High voltage in the heart: PFA catheter design tips from Biosense Webster
Tushar Sharma at J&J Medtech’s Biosense Webster discusses pulsed field ablation (PFA) catheter shapes, sensors, electrodes and device design tips.
Biosense Webster’s pulsed field ablation (PFA) catheter design and development starts in the heart.
That’s where PFA treats atrial fibrillation (AFib) by killing cardiac cells to isolate errant heart signals and prevent them from triggering irregular heartbeats. PFA uses electric fields to permanently open holes in the walls of heart cells through a process called electroporation. PFA catheters need to create lesions through the entire thickness of the heart’s left atrium — which ranges from 2 mm to 6 mm — to completely isolate those irregular signals.
“For pulmonary vein isolation for the treatment of atrial fibrillation, we’re typically looking at a device that needs to give us 6 mm deep lesions on average,” said Tushar Sharma, senior director of medical affairs for Johnson & Johnson Medtech’s Biosense Webster.
Determining that target depth is the first step of developing a PFA catheter, he said, which informs how large to make the electrodes that will deliver the energy to the patient’s heart tissue.
“The reason you have to start from the size of the electrodes is because you need to know — based on the target depth — how much voltage we need, and you need to have the right electrode size to support that voltage,” Sharma said. “[Then] additional parameters like the pulse amplitude, frequency, the time between pulse application and how many pulses are being delivered.”

Biosense Webster’s Varipulse pulsed field ablation platform includes the TruPulse multichannel energy generator. [Image courtesy of Biosense Webster]
The Varipulse catheter — submitted for FDA approval in March 2024 — has 4 mm electrodes with an effective length of 3.5 mm, Sharma said. He declined to divulge the system’s voltage, citing the need to keep Biosense Webster’s proprietary recipe for PFA energy secret.
But he offered some detail on the role voltage plays in ensuring that the cellular electroporation is permanent, while limiting the potential for thermal damage.
Though PFA’s therapeutic effect is nonthermal, the delivery of high voltage through electrodes still introduces the potential for side effects from heat. That requires special consideration when designing the entire system, from the energy generator to the tips of the catheter.
“Everything that is needed to support high voltage, that basically cascades down to individual components,” Sharma said. “That is the biggest difference when it comes to pulsed field versus radiofrequency — you need to have the infrastructure in your devices to support high voltage.”
PFA system designers manage the heat by limiting the voltage that their catheters will deliver, but also by spacing pulses out with enough time to avoid overheating.
“The biggest difference between thermal energies and pulsed field ablation is that the mechanism by which we create a lesion is nonthermal. It is essentially increasing the permeability of the cell membranes, which allows the cell homeostasis to be affected, and eventually, the cells die,” Sharma said. “The key components that we look at from a pulse recipe perspective are voltage and electrode, and also the number of pulses and the number of bursts. You want to keep the pores open, and that is what is going to drive the durability. At the end of the day, the winning recipe is going to be a combination of the right parameters on the pulse that allow us to get an irreversible effect, but then also at the same time the right catheter, the right generator and the right mapping system or the right imaging system that puts it all together.”
A single-electrode catheter will give a cardiologist more versatility for different patient anatomies and the ability to access different areas of the heart. A multi-electrode — or “single-shot” — catheter allows the physician to ablate more tissue at once for faster, less complicated procedures. Device developers are designing their single-shot PFA catheters with different shapes and features, including loops, lattice spheres, balloons, baskets and flowers.
Biosense Webster went with a loop shape for the Varipulse catheter — which can deliver single-shot therapy with all 10 electrodes, as well as more focused energy with just six electrodes— to combine the best features of both types.
“It gives you the efficiency of a single-shot device where you don’t have to make multiple dots to create a circle — you’re still creating a circle,” Sharma said. “But at the same time, it’s not limited to applying it in other areas of the heart, which might be some of the limitations of a flower configuration or a balloon configuration. In terms of electrode spacing, again, it comes down to the spacing between the electrodes — is it consistent, or is it changing? The loop-style catheter really allows us to keep that spacing consistent irrespective of the areas of the heart that we are applying the therapy to.”
PFA catheter materials and striking the right balance

Biosense Webster’s Varipulse pulsed field ablation (PFA) catheter uses laser-cut nitinol tubing for its loop shape, which is adjustable from 25 mm to 35 mm. [Image courtesy of Biosense Webster]
Biosense Webster uses laser-cut nitinol for the Varipulse PFA catheter. Nitinol — which has unique superelastic and shape memory properties — allows the pre-shaped catheter to curl into a contractable loop when deployed inside a patient’s heart.
“Nitinol for us is primarily the shape of the catheter — that’s the backbone of the catheter,” Sharma said.
The Vairpulse catheter’s loop shape can range from 35 mm in diameter to 25 mm as adjusted by the interventional cardiologist using a knob on the catheter handle. Resizing the catheter inside a patient helps ensure adequate tissue contact among varying cardiac anatomies (more on tissue contact later), and also makes it easier to maneuver the catheter inside the cramped left atrium.
But the team needed to strike a balance with the catheter materials and components that are actually conducting electricity — in both directions.
“Remember — and this is a critical component — we’re talking a lot about energy delivery, but this is cardiac electrophysiology, which are electro-anatomical procedures. And so the other component of this procedure is the ECG feedback. … The mapping side of the procedure needs to be supported from the same electrodes that are going to be used for energy delivery, so it’s that right balance of size to support the voltage but also get good signal quality,” Sharma said. “We have the infrastructure and the right balance in our portfolio that … when a catheter is integrated into the Carto mapping system, it’s going to give you good signal quality, it’s going to allow the physician to get ECG feedback, and at the same time have the right voltage and the right electrode size to get an irreversible effect with pulsed field ablation.”
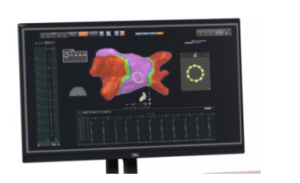
Biosense Webster’s Carto 3 3D cardiac mapping system [Image courtesy of Biosense Webster]
Tissue contact

Biosense Webster’s ThermoCool SmartTouch SF catheter has a contact force sensor to measure tissue contact during cardiac ablation. [Image courtesy of J&J MedTech]
“One thing that you will see front and center of the Biosense Webster portfolio of products for PFA is they all have contact sensing,” Sharma said. “We believe contact is going to be a critical component of the successful recipe for pulsed field ablation.”
The Varipulse PFA catheter features contact indication to tell a physician whether there’s tissue contact for energy delivery.
Biosense Webster’s Thermocool SmartTouch SF dual-energy catheter and the Omnypulse PFA/mapping catheter go a step further with contact force sensing to measure how much force is applied. The SmartTouch SF can toggle between pulsed field and radiofrequency energy, while Omnypulse is a large-tip 12 mm catheter with 12 electrodes for a larger ablation area.
Those catheters have a physical sensor for measuring contact force in grams, while Varipulse can only say if there is or isn’t contact.
“Given the shape of the [Varipulse] catheter, we believe that’s the appropriate approach for a multi-electrode device in our portfolio,” he said.
Advice for device designers and engineers
While this novel technology is exciting, it’s going to take time and continued research to keep innovating and make sure that this novel technology is successful, Sharma said.
“The mathematical equations, the simulations are what get our choices narrowed down,” he said. “From that point on, it’s small tweaks. … You try to assess how close you are to your target, both from an effectiveness standpoint and a safety standpoint — can you give it a little more, can you give it a little less? The majority of it is math, it’s science, but then the fine-tuning of the last bit is a little of evaluating different scenarios.”
Asked for advice he would share with medical device designers and engineers, Sharma identified something that he said has resonated with him his entire career
“Never look at it from the perspective of a single component. … It’s not just OK to have a good catheter, it’s not just OK to have a good generator, always think about an integrated solution because there is a bunch of technology that comes together when a clinical procedure is being performed. So if you are very close and all the time has been focused on just getting the right catheter but if it’s not supported with an integrated solution, there are going to be limitations. Take the time to understand the unmet need. Ask the right people to translate that unmet need if you don’t have the expertise. It’s critical — sometimes an engineer doesn’t quite understand the medical nomenclature or a point the physician is trying to make — so take the time to understand the clinical procedure, take the time to understand the challenges that the physicians are facing, which is their unmet need, and then think about how you’re going to address it. What I have learned in my career is that eventually the solution is going to be an integrated solution. The solution is an integration because you address one component, and then the other part is lagging behind, so your unmet need definition keeps changing. So take the time to understand what an integrated solution looks like.”
“Every novel technology is going to need time, is going to need research, and ultimately the outcomes are going to be where technology meets technique,” he continued. “And so you need to partner not just on the technology, not just on an integrated solution, but also on developing the appropriate workflows — which is the technology aspect — by partnering with physicians and continuing the research.”
Article Source: Medical Design & Outsourcing









