Bioabsorbable polymers for implantable medical devices: What to know
Bioabsorbable polymers degrade and disappear at predictable rates, making them an ideal material for parts of implantable devices that could otherwise impair healing or create an ongoing risk of injury or infection.
Bioabsorbable sutures made of glycolide/lactide polymers, first developed in the 1970s, are strong and flexible enough to hold tissue together to promote healing.
But unlike synthetic sutures which stay in the patient long after a wound has healed, bioabsorbable sutures do not create a long-term risk of foreign-body reactions or require a second intervention to remove.
Johnson & Johnson MedTech’s Ethicon, Medtronic, Braun, Smith + Nephew and other medtech developers offer a variety of bioabsorbable sutures. Ethicon, Medtronic, Gore, Allergan, and Bard are among the makers of bioabsorbable surgical mesh.
Bioabsorbable materials have also allowed the development of devices that must be rigid when they are initially implanted, but become flexible as the natural tissue heals around them.
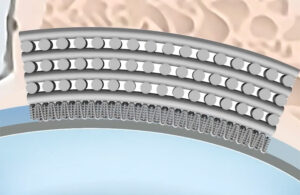
Abbott uses PLLA, a bioabsorable polymer, for its Esprit BTK stent. [Illustration courtesy of Abbott]
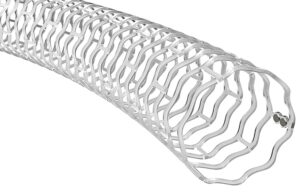
Elixir’s Dynamx bioadaptor is a drug-eluting stent with both metal and biodegradable elements. When it is first implanted, the scaffold is “locked” to establish maximum flow lumen. As the bioabsorbable parts disappear, the structure is “unlocked” to allow the vessel to maintain pulsatility and adaptive flow volume.
Bioabsorbable polymer materials can also create a scaffold to promote the regeneration of the patient’s own cells into new tissue, such as a living, growing heart valve that replaces a defective valve.
Bioabsorbability can also require a compromise on strength or weight.
In 2017, Abbott pulled its much-hyped Absorb BVS poly L-lactide coronary stent off the market after it failed to find a profitable niche in the market. Long-term studies showed Absorb BVS did not improve long-term outcomes compared to drug-eluting metal stents.
Elixir’s Dynamx drug-eluting coronary bioadaptor features a bioabsorbable polymer unlocking mechanism. [Image courtesy of Elixir]
To provide enough radial strength to open a vessel, the Absorb BVS struts had to be thicker than that of a comparable metal drug-eluting stent. Investigators hypothesized that the thicker struts made it harder to deploy and could cause blood-flow alterations and thrombogenicity, especially when the stent was malapposed in the vessel.
Elixir Medical, REVA Medical, Biotronik and Amaranth Medical are among the companies that developed stents made entirely of bioabsorbable materials for the European market in the 2010s.
“[But] after the initial excessive enthusiasm around BVS stemming from the dream of disappearing stents, clinical trials brought a great disillusionment,” according to a review by Mateusz Jezewski, et al, in the 2019 Journal of Clinical Medicine.
Abbott won FDA Approval in April 2024 for the Esprit BTK, an everolimus-eluting, PLLA, peripheral stent to treat chronic limb-threatening ischemia.
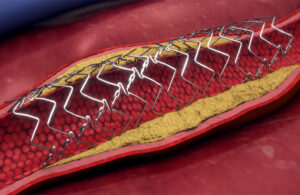
However, drug-eluting coronary stent innovation has mostly shifted to metal stents eluting their anti-restenotic drug from a bioabsorbable coating.
Histological data shows that the polymer that binds the drug to the stent is the main cause of inflammation in vessels treated with metal drug-eluting stents. Metal stents with a bioabsorbable polymer coating can maintain radial strength without inhibiting healing in the vessel wall.

This illustration offers a close-up view of the Synchrony polymer coating on the strut of Boston Scientific’s Synergy BP everolimus-eluting stent. [Image courtesy of Boston Scientific]
Boston Scientific’s Synergy everolimus-eluting stent is coated with Synchrony, the company’s name for a four micrometer-thick layer of poly(DL-lactide-co-glycolide) (PLGA). Each square millimeter holds 1 microgram of everolimus. As the polymer degrades, it releases the drug into the vessel wall to prevent hyperproliferation.
Examples of bioabsorbable materials used in implantable devices
Polymers are the most commonly used bioabsorbable materials for medical devices, though certain metals, ceramics and biologics are also bioabsorbable.
Poly(lactic acid) (PLA) is a group of semi-crystalline thermoplastic polyesters that degrade into lactic acid, a common natural compound. Specifically, PLA is a polymer obtained by the ring-opening polymerization of lactide. Applications include sutures, surgical meshes, dental and orthopedic fixation devices. Drug-delivery systems rely on PLA materials for versatility and biocompatibility.
PLLA (poly-L-lactic acid) and PDLA (poly-D-lactic acid) are homopolymers within the PLA family.
PDLLA (poly-D,L-lactic acid) is a copolymer of PLA.
Polyglycolide or poly(glycolic acid) (PGA) also breaks down through hydrolysis, but the result is glycolic acid. Among other applications, it has been developed as hyperelastic material to promote the development of new bone and for hernia repair.
Poly(lactic- co-glycolic acid) (PLGA) is a copolymer of lactic acid and glycolic acid. It is often preferred for bone substitute constructs because the degradation properties can be controlled by adjusting the ratio between its monomers. PLGA has also been developed for targeted drug delivery.
Polycaprolactone (PCL) degrades slowly and has been used for drug delivery and tissue engineering scaffolds. PCL-based collagen fillers are also used for esthetic applications such as 3D-printed breast implants.
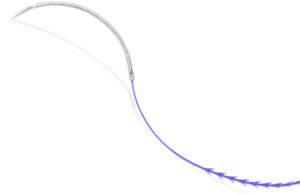
Ethicon’s Stratafix knotless suture uses polydioxanone. [Image courtesy of J&J MedTech]
Polydioxanone (PDO) is a synthetic polymer. It is usually more flexible and weaker than PGA or PLA materials, but it is commonly used in absorbable sutures and orthopedic implants that degrade rapidly over a few weeks. Ethicon’s Stratafix is a knotless polydioxanone suture coated with triclosan, an antimicrobial agent.
Poly(trimethylene carbonate) (PTMC) has been tested for drug-delivery and soft-tissue applications such as ureteral stents because of its unique degradation behaviors, including the production of non-acidic degradation products. Medtronic’s Maxon sutures, first introduced in the 1980s, are monofilament fabricated from a copolymer of glycolide and trimethylene carbonate. Novus Scientific’s Tigr Matrix surgical mesh is a copolymer of glycolide, lactide, and trimethylene carbonate.
Poly(hydroxyalkanoate) (PHA) are a group of biodegradable polyesters produced by either chemical synthesis or bacterial biosynthesis. They are used in a variety of applications, including surgical screws and staples, and in pharmaceuticals for targeted dose delivery.
Poly(L-lactide co-caprolactone) (PLCL) is a copolymer of caprolactone and lactide, combining the strength of PLA and the durability of PCL. It is applied to tissue-engineering and regenerative medicine and may have applications in dynamic environments like vascular tissue. Researchers used PLCL to develop a pediatric heart valve that can grow as the child does.
Poly(ester amides) (PEA) are copolymers of amino acids and dicarboxylic acids offering the thermal and mechanical properties of polyamides, like nylon, with the biocompatibility and biodegradability of polyesters. Applications include drug delivery systems and tissue engineering.
Polyurethanes can mimic natural tissue properties, making them suitable for device coatings to improve the biocompatibility of implanted devices. Polyurethane is also used by Shape Memory Medical as degradable foam in its Impede embolization plugs to obstruct or reduce the rate of blood flow in the peripheral vasculature.
Bioabsorbable or bioresorbable?
The terms “bioabsorbable” and “bioresorbable” are often used interchangeably to refer to devices or materials that degrade and disappear after they are implanted in the body. For example, Abbott’s poly(L-lactide) coronary stent was marketed as the Absorb GT1 Bioresorbable Vascular Scaffold (BVS) system
The terms “bioabsorbable” and “bioresorbable” do not differ in meaning when describing medical devices marketed in the U.S., the FDA told Medical Design & Outsourcing.
Polymer literally means “many mers,” with “mer” originating from the Greek word for “part.” They are a repeating chain of macromolecules – molecules of relatively high molecular mass.
For most polymers, the International Union of Pure and Applied Chemistry (IUPAC) standardized naming convention specifies that the name include the prefix “poly” followed by enclosing marks around the rest of the name. The order of the “locants” in the name indicates the position of each part of the molecule. However, when there is no risk of confusion about the structure, traditional names without the enclosing marks are acceptable.
Bioabsorbable polymer pitfalls and mistakes to avoid
Abbott employees in the testing stage of the Esprit BTK stent development. [Photo courtesy of Abbott]
Engineering devices with these materials requires selecting materials with the desired absorption rate — from weeks to years — and tuning that timetable with the chemistry of the material.
“The reality is that tuning the exact timetable is difficult, so at best you can get ballpark timeframes for absorption,” CytexOrtho co-founder and CEO Brad Estes told Medical Design & Outsourcing. “It’s not just chemical composition either. There are other important variables like geometry and the local implanted environment that also play a critical role in determining absorption rate.”
The performance of bioabsorbable materials can also be compromised by the application of too much heat during the injection process or exposure to heat over time. The materials can also be easily damaged by improper handling or storage.
Bioabsorbables have relatively high viscosity, making them susceptible to shear forces that destroy the integrity of the material during processing.
Molding these materials requires a controlled environment, and the packaging and sterilization of the final product is part of the design for manufacturing process.
Attempts to create bioresorbable implants by replacing metal with a bioabsorbable polymer are likely doomed to fail if the developers do not appreciate differences in the microstructure of the materials.
“We have relied on a nomenclature and anticipated evidence for failure from the metal stent performance and this is not appropriate,” Pei-Jiang Wang of Boston University et al. argued in their analysis of bioresorbable coronary scaffolds. “Failure modes for polymer and metal implants are not the same.”
In a study sponsored by Boston Scientific and the National Insitutes of Health, Wang’s group analyzed the microstructures of poly-L-lactic acid (PLLA) coronary scaffolds. Raman spectroscopy revealed that strain-induced heterogeneity in alignment and crystallinity increased localized degradation, compromising structural integrity that contributes to premature clinical failure.
“Research strategies inherited from metal stents fail to consider polymer microstructures and dynamics [leading to] significant localized structural irregularities that cause asymmetric degradation,” Wang concluded.
Regulatory resources for bioabsorbable polymers
In addition to guidelines for the biocompatibility of implanted devices in general, the FDA recognizes several international standards specific to bioabsorbable materials, including:
- ASTM F2902-16, the Standard Guide for Assessment of Absorbable Polymeric Implants
- ASTM F2502-17, the Standard Specification and Test Methods for Absorbable Plates and Screws for Internal Fixation Implants
- ASTM F2313-18, Standard Specification for Poly(glycolide) and Poly(glycolide-co-lactide) Resins for Surgical Implants with Mole Fractions Greater Than or Equal to 70 % Glycolide.
- ISO 13781, Implants for Surgery — Homopolymers, copolymers and blends on poly(lactide) In vitro degradation testing
- ISO-10993, Biological evaluation of medical devices — Part 1: Evaluation and testing within a risk management process
The FDA also commissioned ECRI to create safety summaries on common materials for medical devices, including the most common bioabsorbable polymers, polyhydroxy acids, PLA, PGA, and other blends and copolymers.
More bioabsorbable polymer know-how and coverage from Medical Design & Outsourcing:
CytexOrtho is developing its ReNew absorbable hip implant using polycaprolactone, a bioabsorbable polymer that is fully absorbed by the body over two years as the patient’s normal tissue replaces it. [Illustration courtesy of CytexOrtho]
- CytexOrtho shares the ‘secret sauce’ for its absorbable hip implants
- Researchers develop implantable heart valve that grows with a child
- Surgical Innovation Associates gains CE Mark for absorbable mesh
- Sharp Fluidics launches port site closure device with bio-absorbable anchors
- European scientists develop absorbable bandages for burn patients
- Texas A&M chemists develop nanoscale bioabsorbable wound dressing
- First clinical use of bioabsorbable vascular grafts in children shows promise
- Athermal laser machining cuts bioabsorbable polymers and more
- Toray touts polymer for regenerative medicine
Reed Miller has reported on the medical device and diagnostics industries since 2000, most recently serving as the commercial/R&D manager for Citeline’s Medtech Insight. Miller lives and works in his hometown of State College, Pennsylvania.
Article Source: MDO