Freudenberg Medical Expands Multi-Layer Extrusion Capabilities
Carrick-on-Shannon, Ireland –Freudenberg Medical, a global developer and manufacturer of medical devices, components, and minimally invasive solutions, announces tri-layer and multi-layer extrusion capabilities from VistaMed, a business unit of Freudenberg Medical, specializing in precision thermoplastic extrusion and catheter manufacturing.

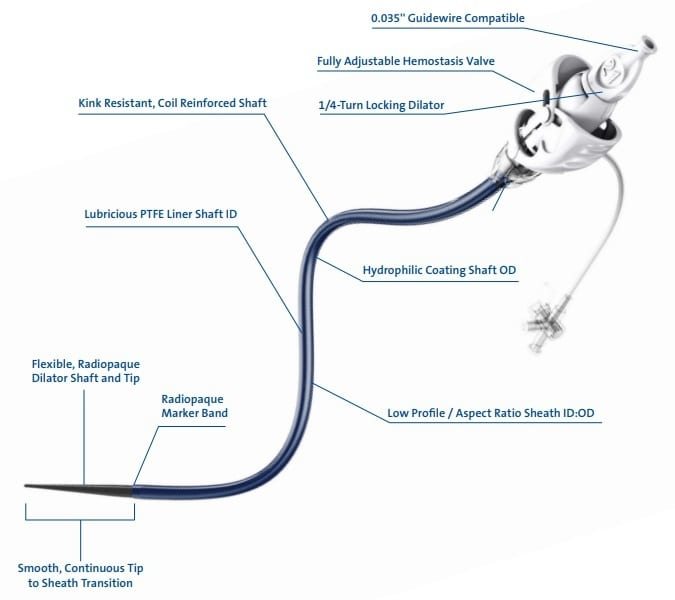
VistaMed’s tri-layer tubing is produced on precision multi-layer extrusion lines in a Class 8 clean room. Designed with a low coefficient of friction, the material on the inner lumen or material layer facilitates the easy passage of a guidewire through torturous anatomical pathways. To ensure that the inner layer of HDPE material has excellent bond integrity to the outer nylon layer, a tie layer material is used in conjunction with refined processing techniques. The outer layer material, typically nylon, is used to facilitate the bonding of balloons or other tubing. A wide range of multi-layer material combinations is available.

“Many years of experience in tubing and catheter manufacturing combined with our continuous investment in the latest process technologies ensures that our capabilities remain best in class to meet the challenging needs of the healthcare industry,” said Patrick Mulholland, Managing Director of VistaMed.
VistaMed produces extruded tubing with OD ranges from 0.2mm (.007”) to 20mm (.787”) and tolerances as low as +/-0.0065mm (0.0002”). Freudenberg Medical’s comprehensive catheter development capabilities include advanced extrusion, handle and shaft development, balloon development and manufacturing, hypotubes and metal micro-components, assembly, and coatings.
About Freudenberg Medical Group:
Freudenberg Medical is a global CMO / CDMO with 11 manufacturing operations and more than 2,000 associates worldwide. Manufacturing capabilities range from high precision molded components and medical tubing to drug coatings, finished devices, catheter shafts and hypotubes for minimally invasive, handheld, and catheter-based devices. ISO 13485 certified quality systems and FDA registered sites are located throughout the Americas, Europe, and Asia. For more information, please visit www.freudenbergmedical.com or email: [email protected]









