Quality Control
-
2021.07.08
The Struggle Is Real: Managing Medical Device Risk
A recent Greenlight Guru survey found that 42% of medical device professionals worry about product risk outside of their work day. Here are some other troubling findings in the report……

Read More -
2021.06.29
Dirty Bronchoscopes are a Bigger Problem Than Ever
FDA received 867 reports related to infections or device contamination associated with reusable flexible bronchoscopes between July 2015 and January 2021. The agency just updated its guidance for reprocessing these difficult-to-clean devices……

Read More -
2021.05.24
How to Achieve Quality through Process Validation
With any manufacturing process, you want certainty in the quality of your products. As you scale production from hundreds or thousands into the millions, you want to know with a high level of confidence that your process produces good parts with consistency……
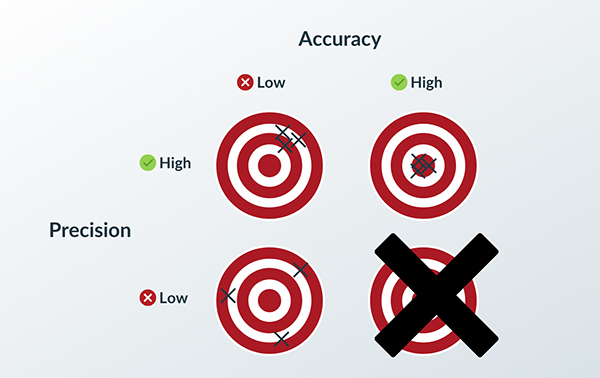
Read More -
2021.05.20
Medtech Needs to Reconsider Its Approach to Cybersecurity
The research was unveiled at the ACC.21 Scientific Sessions. Researchers used Ultromics’s cloud-based AI platform to review the data……

Read More -
2021.04.27
Understanding Regulatory Expectations for Combination Products
Experts from WuXi AppTec Medical Device Testing answer a few questions about submitting combination products for regulatory approval……

Read More -
2021.03.18
Understanding ASTM’s New Specification for Barrier Face Coverings
ASTM has published F3502-21, a standard specification for general-purpose face coverings, which could help consumers understand covering filtration efficiency and more……

Read More -
2021.01.13
Quality Considerations for Drug-Device Combination Products
Understanding the GMP requirements of each component is critical to product and compliance success…….
Read More -
2021.01.12
Compliance Is Compulsory, and Product Labeling Is No Exception
For pharmaceutical and medical device manufacturers, computerized systems validation is vital. The following are three areas businesses must fine tune to ensure computerized labeling systems meet the stipulations of the new GxP regulations”……
Read More -
2021.01.07
Using Appropriate Controls to De-Risk Medical Devices
Strong engineering principles and a strong clinical understanding are of the utmost importance when considering potential failure modes, said a Virtual Engineering Week speaker……

Read More -
2020.12.01
Ensuring Data Integrity and Security in Ultrasonic Welding for Medical Devices
A look at how one equipment supplier upgraded its software to build in data integrity and security safeguards…….

Read More









