Drug Delivery Implant Advantages & Design Considerations
An implantable drug delivery system is a surgically implanted medical device designed to deliver a drug in a controlled fashion directly to targeted tissues or organs. These include polymeric implants, osmotic pumps, microchip-based implants, hydrogel-based implants, implantable pumps, and nanoparticle-based implants.
These drug delivery systems differ from “traditional” drug delivery devices (such as oral or intravenous), which require manual dosing and have been designed to overcome several other limitations associated with those traditional procedures. This blog explores drug delivery implant advantages, implant sites and delivery implementation, and design considerations along with a preview of future directions.
Limitations of ‘Traditional’ Drug Delivery
Traditional drug delivery via oral or intravenous administration is a relatively straightforward approach for many drugs and target indications. However, traditional delivery does have several limitations that include: poor drug bioavailability that necessitates multiple doses or limits therapeutic efficacy; poor patient adherence to dosing schedules; off-target systemic side-effects, such as gastrointestinal irritation; drug metabolism and degradation; the need for trained personnel for administration of intravenous drugs; and the risk of infection at injection sites (particularly for repeated or in-dwelling injection procedures).
These limitations have fueled the development of implantable drug delivery systems.
Advantages of Implantable Drug Delivery
Implantable drug delivery systems enable targeted drug delivery directly to the intended site of action, which can increase localized drug concentration and improve therapeutic efficacy.
In addition, localized delivery can significantly reduce systemic off-target drug effects (ranging from nausea to organ toxicity) that may limit or preclude the use of potentially beneficial drugs. The controlled, continuous release of a drug facilitated by implantable devices allows treatment to extend over prolonged periods without medical intervention.
This is a particular advantage for treatment of chronic diseases, which also eliminates the problem of poor patient compliance. Implantable systems may be designed to meet the specific needs of individuals, enabling personalized medical treatments that are more effective than generalized treatments.
Implant Sites and Delivery Implantation
All implantable drug delivery devices require placement at the target site via a medical procedure, which can vary in complexity depending on the site of implantation.
The implantation site selection depends on a variety of considerations, such as the target tissue or organ, drug pharmacology, and duration of effect required. Common implantation sites for drug delivery systems include subcutaneous (typically on the limb or abdomen), intraocular, brain and central nervous system, bladder, and cardiovascular system (typically within the heart).
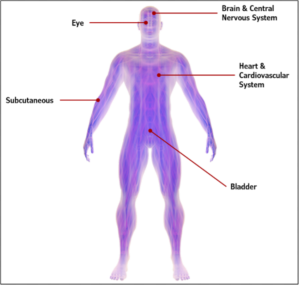
Common sites for placement of implantable drug delivery devices. Photo: StarFish Medical.
An important additional consideration when considering use of implantable drug delivery devices is the delivery of the implant.
Ideally, implantation may be carried out in a non-invasive or minimally invasive manner to minimize overall treatment cost and patient safety risk. To facilitate this, commonly used, off-the-shelf devices or procedures should be the first choice for implantation.
In some cases, a custom companion medical device for implantation must be co-developed. The total time and cost for this should be included in the product development plan.
Implant Design Considerations
Designing implants for controlled drug delivery requires consideration of key issues to ensure safety, efficacy, and compliance are attained. These include the following key factors:
- Biocompatibility—Perhaps the most crucial and difficult design consideration since all implantable devices safety and efficacy profiles will depend on the complex interactions that will occur at the device/body interface. All implant materials coming into contact with the body or body components must be biocompatible to avoid any significant adverse responses subsequent to implantation.
The materials should not cause any kind of toxic response or significant inflammation that would be harmful to the patient. The materials must not provoke an excessive foreign-body response which could negatively impact the essential drug delivery function.
This last point can be a difficult issue to avoid, since some degree of foreign body response is generated by most implants. To minimize the response and reduce the impact it can have on implant drug delivery, careful selection of suitable implant materials, coatings, or surface treatments must be part of the early device design considerations. - Drug potency and stability—any implant design must accommodate the delivered drug’s specific pharmacological properties. The implant must be carefully constructed to ensure the drug’s amount and delivery rate is controlled to permit the intended dosage and dose duration. This can vary significantly from drug to drug, so delivery devices typically need to be customized to the specific drug of choice.
- Drug release mechanism—A variety of options exist for drug release mechanisms, including diffusion,convection, osmotic pressure, and delivery matrix degradation. The appropriate delivery mechanism will depend on a combination of device material properties, drug properties, and the site of implantation.
- Biodegradation rates—For biodegradable implants, the rate of implant matrix degradation must be matched to the defined drug release rate. All compounds generated by the degradation process must be biocompatible to avoid any negative implant site responses. In this sense, the implant materials must be biocompatible and the by-products produced as the material biodegrades.
In contrast, non-degradable implants must be designed for easy removal once the drug payload has been delivered to avoid any potential long-term implant-associated complications. A non-degradable implant may contain or produce fewer compounds that can impact biocompatibility than biodegradable ones, making the initial biocompatibility assessment simpler and less costly. The potential for longer term implantation can necessitate long-term, costly biocompatibility testing. - Implant size and shape—In general, the smaller the implant, the better in terms of ease of application, patient comfort, and implant site accessibility. However, the implant must be appropriately sized to carry the required drug payload.
The implant shape is important to facilitate efficient transport into and out of the device. This can become especially important in the case of implanted cells that express a therapeutic drug in vivo and require the transport of nutrients into the device to maintain cellular viability.
Implant geometries should avoid sharp corners that may provoke a mechanically-initiated foreign-body response that can negatively impact drug delivery over time. - Patient compliance—Implant design should greatly simplify patient compliance by minimizing the frequency of administration and removing all manual dosing requirements.
- Manufacturing considerations—The design should consider methods of manufacturing to avoid overly complex fabrication or assembly techniques and ensure maximum consistency and product quality. As these implants are generally combination products consisting of both a device and drug component, the associated regulatory requirements for both components must be considered during the design and manufacturing processes.
In addition, appropriate processes, equipment, and facilities must be used to ensure product safety, including sterilization, packaging, and storage. These include use of controlled spaces such as cleanrooms or isolators and controlled storage environments to avoid product degradation or contamination.
In this regard, it is crucial manufacturing facilities are maintained within a robust, audited quality system that is fully compliant with the appropriate standards and certifications, particularly related to environmental monitoring and bioburden control.
Future Directions
Research in this exciting field continuously improves on existing designs and materials. Novel biodegradable polymers improve on biocompatibility and expand on drug delivery profiles. New smart materials that respond to implant environment changes or stimuli to more precisely control drug delivery are under development.
Traditional areas of drug delivery are being combined with new treatment modalities such as gene and cell therapies to further extend and enhance their therapeutic possibilities. New manufacturing techniques miniaturize devices and expand the range of sites for implantation within the body.
Minimally invasive techniques for implant placement are being adapted from related surgical procedures to improve patient comfort and adoption. Considering all of these exciting new developments, the future of implantable devices for drug delivery is very bright.
Source:MPO









