Multimillion-Dollar Grant Awarded to Realeve for Post-Stroke Recovery Study
Company’s technology has already been clinically proven in more than 700 patients for treating a brain-related disorder.
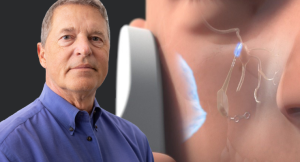
Realeve Founder Peter Bonutti M.D., introduces breakthrough micro implant, clinically proven in 700+ cluster headache patients for treating stroke, Alzheimer’s, and other brain diseases. Image courtesy of Business Wire.
Realevehas received a multimillion dollar grant to support a Cleveland Clinic study focusing on post-stroke recovery.
The trial will use Realeve’s Pulsante micro-neuromodulation system, the first externally powered implantable neuromodulation device that stimulates the sphenopalatine ganglion (SPG) in conjunction with hyperbaric oxygen to potentially enhance blood circulation in stroke patients, thus minimizing brain damage and optimizing neurological recovery.
Realeve’s technology has already been clinically proven in more than 700 patients for treating a brain-related disorder. Realeve has also been granted U.S. Food and Drug Administration (FDA) Breakthrough Device designation and is working to obtain FDA approval and CE Mark to treat cluster headaches in 2024. Breakthrough Device designations are granted by the FDA to expedite the review of technologies that can improve treatment for life-threatening or debilitating conditions.
“We are excited to leverage the power of this external implant that has been clinically proven with 700+ patients in enhancing post-stroke and cluster headache recovery. We are also exploring its applications in other central nervous system disease conditions and excited about the potential,” Realeve Founder/President Dr. Peter Bonutti said.
Realeve’s ultimate goal is to solve one of the critical remaining barriers in brain health—bypassing the brain’s natural barrier to deliver drugs for stroke, cancer treatment, and other degenerative orders. Realeve is evaluating the potential effects of its patented SPG therapy on the Blood Brain Barrier (BBB) using its clinically proven Pulsante System. The BBB functions as a vascular gatekeeper to the central nervous system (CNS), governing the passage of medications and therapeutic interventions into the brain. This technology holds promise for addressing conditions like Alzheimer’s, tumors, and other central nervous system disorders. The convergence of these therapies is a potentially significant advancement in treating neurological diseases of the brain, and a compelling opportunity for investors in the biotech sector.
Pulsante consists of a miniature bioactive implant inserted orally using a minimally invasive technique that leaves no visible scars. It pairs with an external, hand-held controller that stimulates the SPG, which controls the autonomic nervous system and can impact multiple neurological conditions. The targeted therapy provides both pain relief, and can increase blood flow and medication delivery to the central nervous system.
Additionally, Realeve has solved a key challenge with surgical implants—powering them. Most implants such as pacemakers, cardiac and insulin pumps, etc. require batteries. These batteries have many challenges, the biggest is that batteries wear out which requires an additional surgery. The Pulsante handheld remote control wirelessly powers the implant and allows for two-way communication and wireless updates to the system.
“Realeve is focused on optimizing therapies to the central nervous system. With a commitment to innovation and a patient-centric approach, the company aims to improve the lives of individuals with neurological conditions through stimulating extra cranial nerves. By developing novel technology, Realeve strives to provide effective, targeted relief that can make a lasting impact on patient well-being,” Realeve Advisory Board Chairman Dr. Frank A. Papay, M.D., chairman of the Dermatology and Plastic Surgery Institute, and Cleveland Clinic professor, Lerner College of Medicine at Case Western Reserve University.
Papay is an inventor of technology that Cleveland Clinic licensed to Unity HA, the parent company of Realeve. He serves as a consultant to the company, for which he received equity.
Realeve is a medical device company developing treatments for diseases and central nervous system conditions through the control of the SPG via the Pulsante micro-neurostimulator system. Founded in 2019, Realeve builds on more than a decade of proven efficacy of the Pulsante system.
Article Source:MPO









