On the Rise: Wearable Injector Systems
The drug-delivery industry is rapidly changing, with innovation often outpacing manufacturers’ ability to produce and release new products. The motivation for these changes has always been patients’ desire for more freedom in their treatment plan. Long gone are the days where a patient needs to schedule an office visit to discuss or adjust their drug regimen: the advent of automatic drug-delivery devices has empowered patients to take their treatments in their own hands. Still, the amount of effort involved and the potential for error associated with traditional drug-delivery methods leaves room for further improvement.
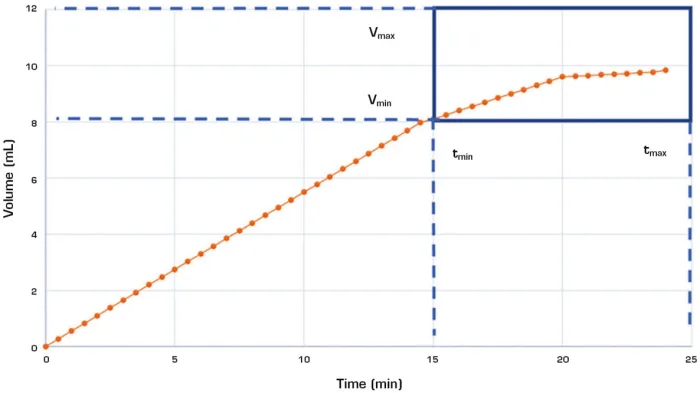
Fig. 1 – This chart shows an example of a drug-delivery dose profile. Dotted lines help visualize the bounds for maximum and minimum delivery dose and time.
Wearable injection systems are drug-delivery devices that adhere to the body, automatically delivering set doses to the patient at optimal times. In a society that expects everything to be instantaneous, wearable injection systems represent the natural evolution of chronic disease management. The development of these devices has been spurred by the concept of patient-centricity and an increased focus on human factors in drug-delivery design. The trend toward the usage of biologics in pharmaceutical development has also contributed by necessitating the development of delivery systems equipped to handle high-viscosity solutions. As wearable injection systems have emerged in response to these conditions, so has a completely new set of challenges regarding device design and testing.
DEVICE DESIGN
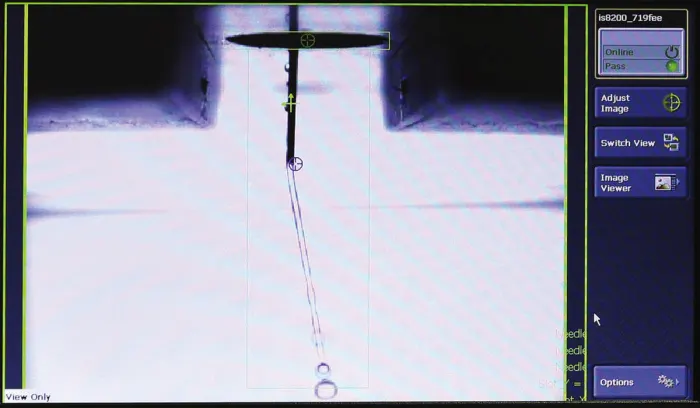
Fig. 2 – This monitor shows the image taken by an optical micrometer, determining the insertion depth of a rigid needle during a test.
When addressing the use case for these products, it was clear to manufacturers that a gap existed in the marketplace. On one hand, prefilled syringes (see Figure 2) and autoinjectors are not optimized for delivery times longer than 20 seconds, and at the other end of the spectrum, devices like insulin pumps are overly expensive and complicated for periodic fixed volume drug delivery. Wearable injection systems are designed to fill both gaps, enabling comfortable, low-profile delivery of precisely dosed medications over fixed or variable periods of time. The evolution of this product has included the following five components:
1. Adhesive Patch. This component represents a unique design challenge for device manufacturers. Adhesives are not a component found in many previous drug-delivery devices, requiring manufacturers to acquire a new set of skills and expertise. The adhesive properties need to be optimized for patient comfort during use and removal of the product, and the adhesive patch also needs to be designed in a way that ensures proper orientation and skin contact are maintained during the delivery process.
2. Device Body. The device body houses all internal mechanical and electrical subcomponents of the system. The main purpose of the mechanical components is to dispense the drug to the body at either a set or variable flow rate. Certain devices have controllers that can define the flow rate, either through physical means on the device or through a paired application. The flow rate can either be a user preference based on comfort, or a physician requirement. The electronic subcomponents typically allow for communication with external devices to assist the patient with tracking their progress over time. It is also common to have a viewing window and audio/visual feedback to alert the patient when the delivery is complete.
3. Drug Cartridge. The cartridge is where the required drug volume or bolus is held. This generally consists of a glass container, a rubber stopper, a rubber seal, and an aluminum crimped cap. The break-loose and glide force for the stopper needs to be evaluated to properly design the actuation mechanism.
4. Activation Button. The device will generally have a clearly marked button for starting the injection. There are many factors that need to be considered in the button design, including tactical feedback, activation force, and design features to prevent accidental actuation.
5. Needle/Soft Cannula. While a stainless-steel needle is most commonly used, a soft cannula can instead be used in cases where the delivery time may be longer than 20 minutes and patient comfort is a concern. The needle and soft cannula require similar evaluations determining the insertion depth and lockout confirmation. As in all drug-delivery systems, sharps protection is critical, requiring a passive mechanism to hide the needle after injection.
WEARABLE DEVICE BENEFITS
Wearable injection devices come with a wide array of benefits to the user and drug manufacturer beyond the simplicity of the device. The device’s functionality provides increased opportunities for delivering both larger doses and higher viscosities, and in some cases both. This is accomplished while mitigating discomfort to the patient associated with larger doses, and in many cases reducing the total number of injections required. In situations where drugs are delivered intravenously over long periods of time, as is often found in the treatment of cancer or auto-immune diseases, these products can allow for delivery in the patient’s home rather than a clinical setting. Limiting unnecessary time in hospital or clinical settings also reduces the risk of hospital acquired infections (HAIs).
Wearable devices also introduce increased opportunity for connectivity between digital health platforms and the devices. The design of these products can allow for additional electronics to be built in, offering an unparalleled level of digital control and oversight. Through connected applications, the patient can adjust the deliverable volume or flow rate depending on their preference or physician suggestions. In the case of diabetes care, the devices can even interface with other external devices like glucose meters, actively monitoring glucose levels to adjust the insulin delivery profile. These closed loop systems represent a tremendous leap forward in utilizing machine intelligence to help patients. Providing greater treatment visibility can also improve patient outcomes in many cases, allowing physicians to catch potential issues before they occur.
PRODUCT TESTING
Along with this myriad of benefits also comes a set of challenges for drug-device manufacturers and designers related to testing, which are currently addressed primarily through internal specifications and procedures. International standards organizations have only recently begun to address the need for well-defined criteria for testing wearable devices.
The International Organization for Standardization (ISO) released a draft subsection of ISO 11608 to address the unique requirements of these devices, referred to in the standard as On Body Delivery Systems (OBDS). This definition is more than likely kept purposefully broad in anticipation that these devices will continue to develop in terms of total output and design variations. In order to meet these growing requirements, manufacturers need universal testing systems flexible enough to perform the wide range of tests outlined by standards and internal specifications.
The most critical test criteria are related to the dosing of the device. Like more traditional devices, dose accuracy and dose volume are measured, but for OBDS the ejection profile must also be characterized. The ejection profile helps visualize the drug delivery over time, and it often has specific limitations based on the drug type and viscosity. The graph in Figure 1 shows a sample of scale data for a typical test with functional bounds on the dose delivery time and volume. A precision scale is required to capture the dispensed volume after the device is activated. To capture the profile, the data capture rate of the scale is a critical parameter needing enough data points to properly plot the curve. This an important distinction from pen injector tests, where the final volume is captured along with the start and stop time for the delivery.
When it comes to the activation and completion of the ejection, a range of tests are needed. The activation force is measured using a universal testing system to simulate the pressing of the button. Different button designs may be evaluated to find the optimal activation force based on the 5th to 95th percentile of hand strength for the target patient. Being able to detect all device feedback is important, requiring test systems capable of capturing audio and visual cues of activation as well. Auditory sensors can be added to perform click detection, syncing the events up with the scale and force data to characterize the event. Integrated cameras should be utilized as well to confirm that visual cues have occurred, including the color change in the viewing window of the device or any light emissions.
Evaluating the adhesive properties of the patch can be broken down into tack, peel, and shear tests. Existing ASTM and ISO standards can and should be used as building blocks for developing these test methods. The main difference should be the inclusion of additional test environments and preconditioning to better simulate the real-world usage of these products. Many adhesives can strengthen over time, a property that can cause pain for the patient at removal. Creating time studies for adhesive tests is a guaranteed way to avoid these issues. Beyond time studies, adhesives should be evaluated after experiencing use-case conditioning. This can include axial torsional cyclic loading to replicate the stretching and twisting of the skin or the application of water or lotions to the substrate prior to removing the adhesive. The change in adhesive properties with respect to the preconditioning can assist with identifying adhesives able to withstand continued patient use.
Finally, evaluating the needle or cannula insertion distance is especially important for a wearable device. Patient movement could cause small variations in insertion depth throughout the delivery, meaning the optimal insertion range must be validated on the system. Optical micrometry systems can be integrated on universal test frames to accurately determine needle extension past the device housing.
CONCLUSION
It is imperative to create a robust design evaluation process for developing the next generation of injection devices. Identifying universal testing systems that can efficiently evaluate all the product requirements and simplify data analysis is a key part of this process. Based on current trends, the global demand for wearable injectors will continue to rise, meaning that both R&D and quality labs will need more and more testing capabilities. In response to these needs, equipment manufacturers will need to further specialize their test systems, working collaboratively with global device manufacturers to stay ahead of the curve.
Article source: Medical Design Briefs









