Big Prospects for Medical Device Miniaturization
Strides in medtech miniaturization have enabled surgeons to reach remote areas of the body and have also made healthcare more accessible through portable and wearable medical devices. But while much has been accomplished, there are still potential new gains. There are also challenges to overcome as medtech innovators continue to shrink the state of the art—but there are solutions.
To demonstrate just how long the trend toward miniaturization is, Raghu Vadlamudi, chief research and technology director for Donatelle, says his company has been making micro components for 50 years. “Pacemakers were pretty big at one time—and now pacemakers can be implanted directly into the heart,” he tells MD+DI, pointing to Medtronic’s Micra. “Efforts are in place to miniaturize medical devices out there for cardiac rhythm management, wearables, and even orthopedics. And also for minimally invasive surgery.”
Miniaturization is the niche to be in, according to Donna Bibber, CEO of Isometric Micro Molding. Last year, Bibber was a member of the MD&M East 2021 panel discussion on molding, “Solving Today’s Challenges in Injection Molding for the Medical Device Industry.” During the discussion, Bibber told the audience that miniaturized medical devices enable such advances as natural orifice transluminal endoscopic surgery (NOTES) and other minimally invasive surgeries. They are also necessary for cardiovascular and neurovascular procedures as well as drug delivery.
Scott Thielman, CTO of Product Creation Studio, says that “unobtrusive access is the major opportunity for healthcare innovation. But access to what and for whom depends on where you look. In the surgical suite the ability to reach and manipulate distal tissues more efficiently with fewer surgical openings continues to drive R&D into miniaturized interventional tools. In other hospital units there is a drive to bring more capabilities to the patient’s room to monitor, image, and diagnose at the point of care rather than be gated by a central lab. To help us avoid healthcare facilities altogether, there is a drive to shrink monitoring, diagnostics, and therapies to fit into our homes, our phones, and onto our bodies. Different scales, environments, and users but seamless access is the opportunity.”
Thielman, too, points out Medtronic’s Micra, noting that this pacemaker “can be delivered via a catheter, something unimaginable several decades ago.”
Such gains in cardiovascular devices are now inspiring new frontiers for miniaturization. “Cardiac surgical procedures have saved countless lives by stenting, valving, suturing, and pacing,” Thielman says. “As we move those procedures to the brain, we deal with even smaller and more delicate vasculature and neurons. Things that fit down a 6 French lumen suddenly must fit down a 3 French lumen or smaller. And the desire to manipulate and treat within these tighter confines continues to drive miniature mechanism feasibility studies.
“In related developments the desire to implant less obtrusive devices within the body has driven innovations in power management and delivery. And we are seeing implantable solutions for everything from pain management to sleep apnea,” he adds.
Thielman also believes that point of care is the other area in which miniaturization “enables more distributed access and personalized care. We are seeing point-of-care imaging ultrasound really gain traction, case in point is the Philips Lumify system. Also, the multifunctionality of devices can mean fewer boxes in a crowded care environment; consider the VOCSN solution from Ventec Life Sciences.”
He shares a recent project of Product Creation Studio’s that demonstrates future advances in miniaturization. “We have been supporting the innovation team at Wetware Biosystems to miniaturize a transcranial magnetic stimulator for the potential treatment of traumatic brain injury due to exposure to concussive pressure waves found near a battlefield explosion. Their goal is to embed this novel therapy within the soldier’s helmet, enabling potential treatment immediately, when it does the most good. Our collaboration has created a therapeutic pulse system that demonstrates the feasibility of the concept.”
What Does Miniaturization Actually Mean for Medical Devices?
When it comes to micromolding, the smallest parts Isometric makes are “one thousand parts per plastic pellet,” Bibber explained. They also make parts that are not quite so small, such as 1-in. parts with 3-micron channels and large parts up to 6 in. with micro features, thin walls, and tight tolerances. Such part and feature sizes mean that Isometric is working with “single-micron tolerances,” explained Bibber.

Miniaturization could also be seen as “doing more in the same space or more in less,” describes Aaron Johnson, VP of Marketing & Customer Strategy for Accumold. And that entails “adding higher functionality for patient comfort and care, such as adding functionality to surgical tools or to collect data for better patient experiences, as well as for home or mobile care. For instance, a lot of devices are competing for real estate on your person—so if you are wearing it, why can’t it do more?”
Consider the expanding functionality of today’s smart watches, he says. More sensors are often being added in the same amount of space or smaller than a previous version for more monitoring, he says.
When pushing the limits to go smaller, Johnson says it is important that the project satisfy three pillars: Capability (can it be made), Scalability (can it be made at commercial volumes), and Sustainability (will manufacturing partners be around to support the product long term).
“There are really cool innovations going on in our customer R&D labs—but will it get to commercialization? That’s never been a more important question,” says Johnson. “There are a lot of different components that can come from different suppliers—along with material and geometry—they all must scale down together,” he says.
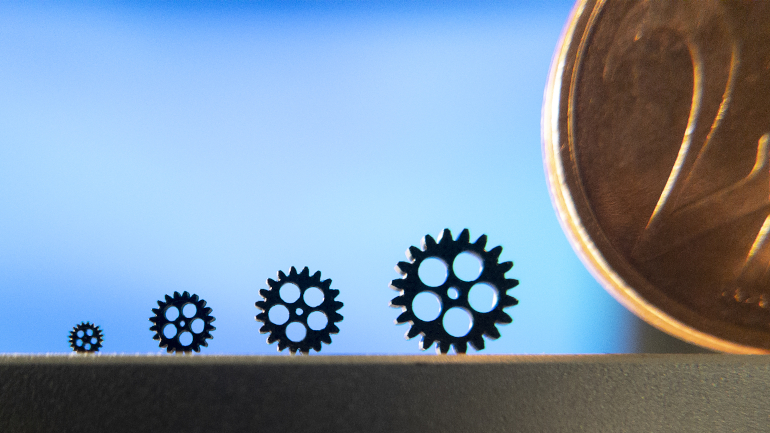
Prototyping and Achieving the Once Impossible
The “goal of prototyping is to get to commercialization,” Johnson explains. But there can be challenges.
Don Baumgarten, Product Creation Studio’s director of mechanical engineering, suggests a way to approach prototyping. “Building miniature device prototypes at 1:1 scale can be time-consuming and expensive because many components must be ordered from highly specialized suppliers,” he told MD+DI. “To speed up development and reduce costs, it often works well to build large-scale prototypes first, such as 2:1 or 5:1 scale, so that components can be fabricated using standard machining and manufacturing processes. The goal is to refine the design as much as possible at the larger scale to minimize the number of design iterations needed at actual size.”
Johnson notes that there are “huge advances in prototyping are shortening the gap between product development and manufacturing.” For instance, “it’s been a long-standing challenge to find high-precision, rapid 3D printing,” he adds, but his team has had success using Nano Dimension’s Fabrica Digital Light Processor (DLP) system to produce micropart prototypes. “It has incredible resolution and prints up to 1-micron layers.” He adds that other systems potentially useful for microprinting include Boston Micro Fabrication’s Projection Micro Stereolithography (PµSL) technology and Nanoscribe’s laser lithography technology.
Accumold is using such additive manufacturing technology to produce prototypes as well as digital rapid-printed tooling instead of steel tooling. “We can use the printing technology to make the cavity for molding,” Johnson says.
When printing prototypes for eventual injection molding production, “printing doesn’t necessarily tell us that something is moldable, but we can confirm if it will fit the design,” he says. “DFMM [design for micro molding] helps us determine if it is moldable.”
DFMM is “very specific to a material, machinery, and tool making,” he expands. “That’s why it is important to partner with someone with a long history of micro molding. For example, while material selection has a profound effect on the ability to manufacture a part, it usually comes down to experience in working with the material rather than some data sheet—the experience helps you save time and resources in getting it right first.
“We are always helping customers push limits in terms of size, thin walls, and accuracy and helping solve something never done before. And micro molding is just one part of miniaturization—there are other components and they all need to fit together. We can handle some light subassembly and may be able to suggest some part consolidation, which could benefit the project,” says Johnson. “We like to start at the napkin stage with companies and help them dream big—you never know—the impossible might be possible.”

Optimizing Micro Designs for Medical Devices
For product development, Donna Bibber shared during the MD&M East panel discussion that Isometric uses CT scanning as a method for Design of Experiments. “It’s basically an x-ray. You would put your very small parts in Styrofoam and rotate them in front of the x-ray. As they are rotating, it is creating a point cloud, and you get a shell of the part that can lay over the solid model and determine the differences not only of the drawing dimensions, but all surface dimensions in fractions of a micron. Within 15 minutes of scanning 16 cavities, we can tell all the differences of the CTQs and what we did in our DOE at the molding machines,” said Bibber. “We create combinations of high pressures, speeds, and temperatures and low pressures, speeds, and temperatures . . . . With all this data, we can build up assemblies using real-time data of all those different components—a full factorial, if you will, for developing our Cpk [process capability index] right at the modeling machine. . . . It is fantastic to have all that information to feed back into the process.” Bibber said that this approach supports faster time to market “because your timeline for product development can be minimized.”
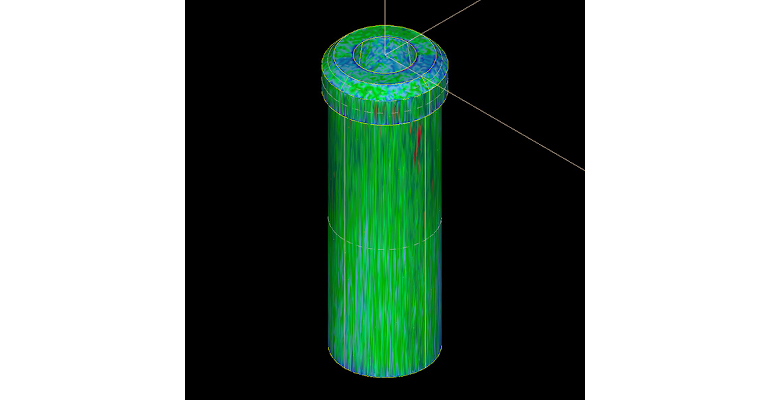
Bibber also added that another output to this approach is an STL file that can be put back into the finite element analysis assumptions. In addition, Bibber expressed that the real-time data and dimensional data images can be “a fantastic way” to support clinical submissions.
CT scanning can also help with achieving the targeted Cpk. “You still need to achieve a 1.33 Cpk when working with single-micron tolerances, so it is important to look at pFMEA with a new set of eyeglasses,” Bibber said. During the MD&M East panel, she explained one project that involved +/-8 micron tolerances. Isometric built the tooling to 20% of that tolerance, so there would be 80% left to take up in the molding, gage R&R, the material lot-to-lot variability, and material drying that takes up a portion of the tolerance to reach a 1.33 Cpk. Isometric also looks at environmental conditions, because materials, such as hygroscopic resins, can change in size. “We call it our Microns Matter process,” Bibber said.
Manufacturing the Miniature
Working at such a small scale is not without its challenges. “We manufacture parts a tenth of a milligram—you can’t see them with the naked eye,” says Vadlamudi of Donatelle. “You need to be able to understand the magnitude of these processes and control the manufacturing processes to produce these parts consistently.”
As parts get smaller, “you need to employ more-stringent processes,” Vadlamudi says. “Equipment has to change. [Machines] need to be suited to manufacture smaller parts, such as 14-mm extruder screws with plungers of 5 mm or less, for instance.
“Materials also have to be handled properly,” he adds. In injection molding, for instance, “you have to dry materials before molding. But how do you dry material in such small quantities?” Also, static control is a concern during manufacturing and packaging, he says. “We are innovating as we encounter these challenges.”
Donatelle’s approach entails manufacturing miniature components and some subassemblies in workcells, integrating all processes as much as possible. “We used to machine a tiny part with tiny holes and then take it to a different machine to drill the holes, but now we integrate machining with laser drilling and other processes right in the workcell. We can also integrate metal injection molding as well as inspection and packaging,” he says. Metal and plastic manufacturing processes typically would be handled in different workcells, but plastic and silicone manufacturing could be integrated in one cell. “You can mold and overmold in the same machine,” he says.

Every workcell has a microscope to inspect plastic part features as small as 30 microns, Vadlamudi explains. “You can imagine the difficulty in handling these parts—they can’t be touched by human hands. Nothing comes out of these workcells until ready for shipping,” he adds.
Robotic technology is also becoming integral parts of such workcells, he adds. “Industry 4.0 will become more prevalent in the near future. Every machine is a computer now, and they can all communicate with each other.” For instance, he describes how controlling the water temperature during molding is critical. All processes can now be hooked up together so that operators can see where a problem might occur.
Industry 4.0 allows more visibility into manufacturing, inspection, preventive maintenance, and more, he says. “All of these can be automated to prevent incidents that could stop production,” he says.
Micro parts need custom manufacturing and packaging processes, so Donatelle designs systems to be component specific. These products typically aren’t manufactured in high volume, he says, so Donatelle designs the workcells so that they can be restructured and reconfigured, almost every day. “We could be building an implant in the morning and then a surgical device that afternoon,” he says. “We work with quite a few different manufacturing processes under one roof, and we can work with customers during the concept stage and help with material selection and the manufacturing process.”
Article source: Qmed









