Explore the critical role of electronics cleaning validation in medical device manufacturing. Learn about regulatory requirements, contamination risks, and effective cleaning methods
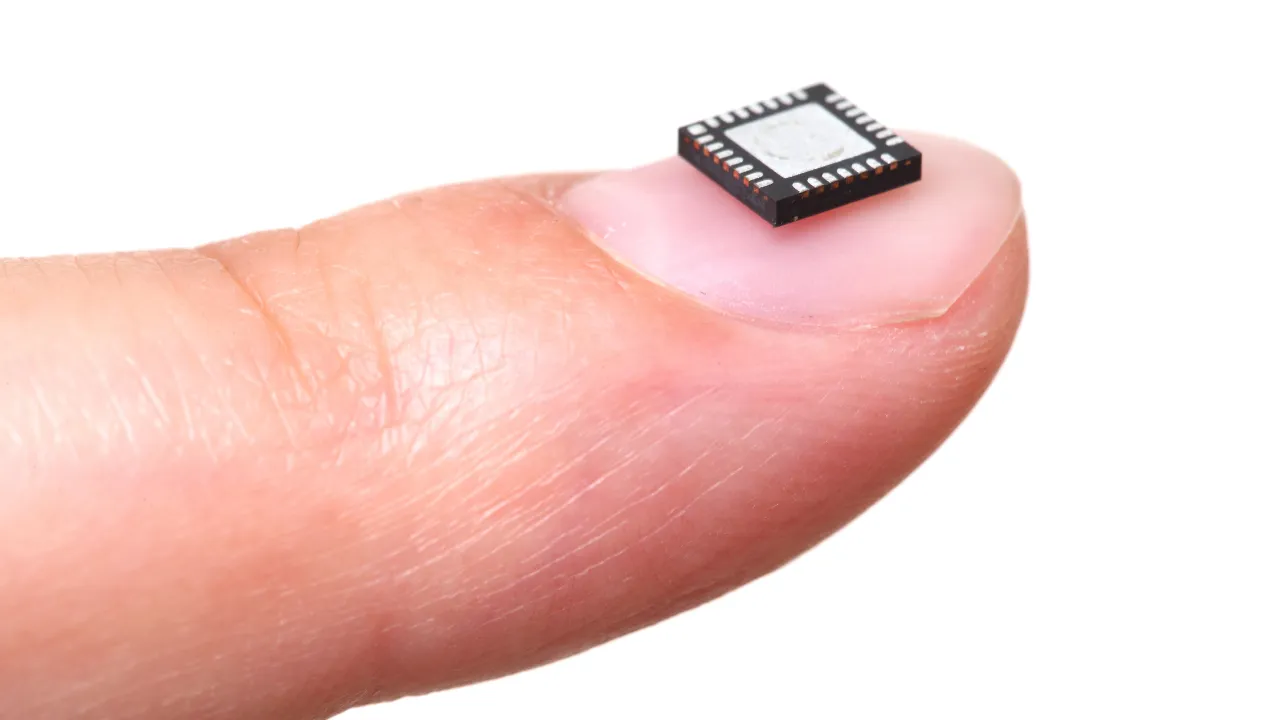
As electronic components become more sophisticated and miniaturized, the cleaning processes used during manufacturing are a crucial element in validation protocols.IMAGE COURTESY OF
At a Glance
- Vapor degreasing emerges as a superior cleaning method for complex medical electronics, ensuring regulatory compliance.
- Proper electronics cleaning validation is critical for patient safety as medical devices become increasingly miniaturized.
- Structured IQ/OQ/PQ validation framework essential for medical device manufacturers facing heightened regulatory scrutiny.
Medical device equipment manufacturers work in an environment where product quality isn’t just a competitive advantage, it’s a regulatory necessity with direct implications for patient safety. As electronic components become more sophisticated and miniaturized in modern diagnostic instruments and therapeutic devices, the cleaning processes used during manufacturing are a crucial element in validation protocols that ensure device safety, reliability, and regulatory compliance.
While electronics cleaning has long been part of medical device manufacturing protocols, its role in the validation framework has gained increased attention as regulatory scrutiny increases and devices become more complex. Regulatory inspections and audits now often examine cleaning validation documentation in detail, recognizing that even microscopic contamination can affect the performance of today’s advanced devices.
Regulatory framework and compliance requirements
Medical device manufacturers must follow a number of standards and regulations, for example, FDA 21 CFR Part 820 Quality System Regulation and International Standards Organization (ISO) 13485 standards, both of which establish explicit requirements for process validation. These regulations require validation of any process whose output cannot be fully verified through subsequent inspection and testing, a category that includes electronics cleaning.
FDA’s guidance on process validation emphasizes that manufacturers must establish documented evidence that cleaning processes consistently remove potential contaminants to predetermined specifications. Similarly, ISO 13485 requires validation of processes where the resulting output cannot be verified by subsequent monitoring or measurement, making cleaning validation mandatory for most electronic medical devices.
Cleaning validation documentation becomes particularly important during FDA inspections and notified body audits, where regulators will examine whether manufacturers have properly established, controlled, and monitored their cleaning processes as part of the overall quality system.
Understanding contamination risks in medical electronics
Electronic medical devices face specific contamination challenges during manufacturing that can compromise functionality and reliability if not properly addressed. Common contaminants include:
–Flux residues from soldering operations can create unintended conductive pathways on circuit boards, potentially causing current leakage, signal interference, or even short circuits over time.
–Particulate matter such as dust, fiber, and metal fragments introduced during assembly can lodge between components, creating potential points of failure or interference with mechanical functions. In microelectronics applications, even microscopic particles can bridge circuits or obstruct connections.
–Oils and fingerprints from handling during assembly can attract dust and potentially promote corrosion on sensitive components. These residues may not cause immediate failures but can degrade performance over time, particularly in devices with expected lifespans of many years.
–Ionic contamination, which includes residual salts and other ionic compounds, presents a particular concern for medical electronics. When combined with even minimal moisture, these contaminants can initiate electrochemical migration and corrosion processes that progressively damage circuitry.
For critical care devices, in vitro diagnostic instruments, point-of-care testing equipment, and implantables, these contaminants represent significant risks to device performance and reliability. The consequences of contamination-related failures range from erratic readings in monitoring equipment and false diagnostic results to complete failure of life-supporting devices, potentially compromising clinical decisions and patient outcomes.
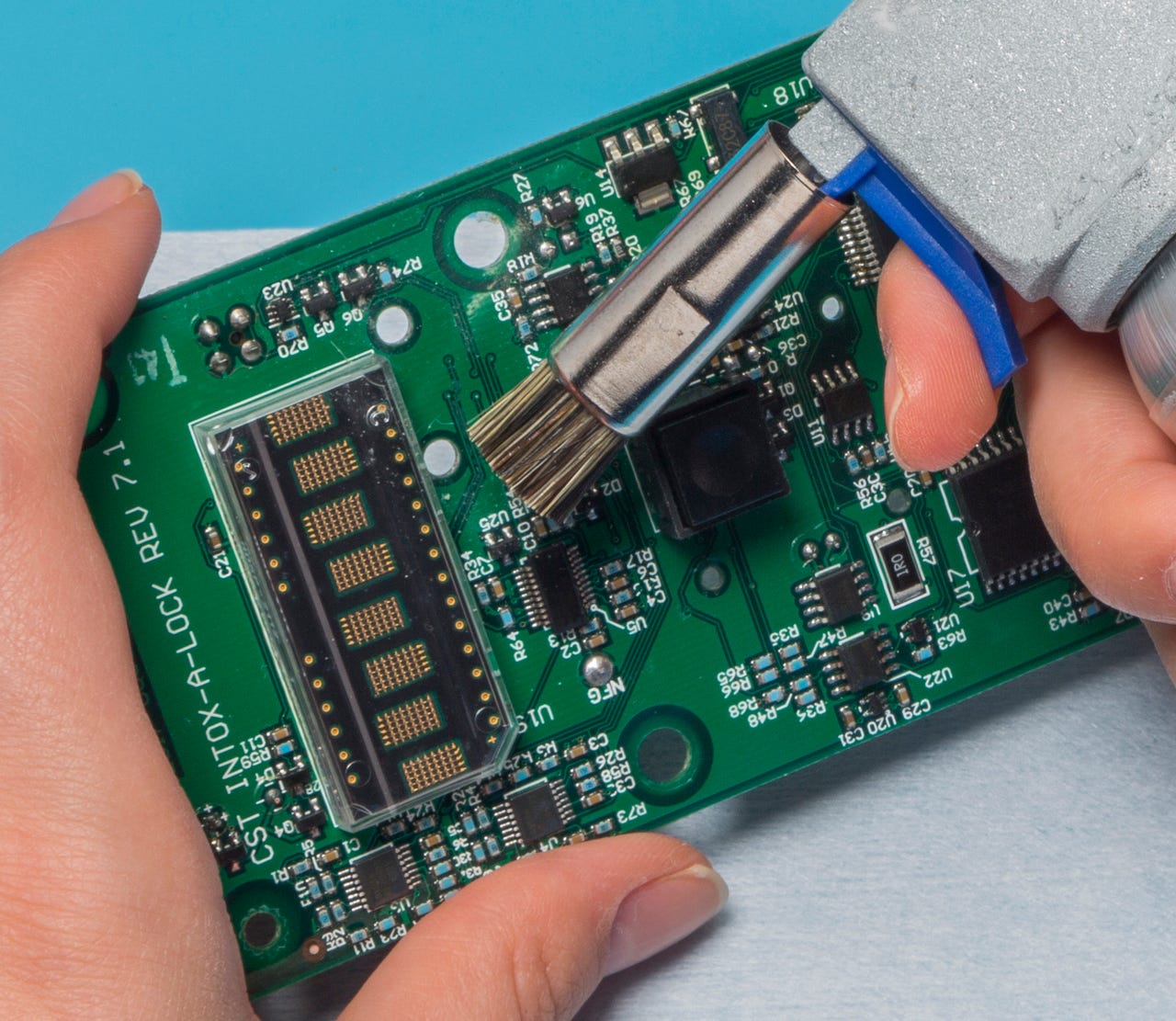
Selective, or spot cleaning techniques require precise application and careful validation to ensure that protected areas stay uncontaminated by cleaning agents or displaced contaminants. Image courtesy of MicroCare.
Effective cleaning methods for medical electronics
Several cleaning methods have proven effective for medical electronics, each with specific advantages depending on device complexity, part sensitivity, and contamination type. However, vapor degreasing is increasingly standing out as a reliable and efficient solution for cleaning delicate electronic medical devices.
Vapor degreasing is one of the most effective methods for cleaning medical electronics, offering several advantages. It uses the condensation of solvent vapors to clean electronic components, effectively removing oils, greases, and flux residues from intricate and sensitive assemblies. This method excels at cleaning parts with complex geometries, small features, hard-to-reach areas, and those made with a combination of metals, plastics, and delicate substrates which are common in electronic medical devices.
Modern vapor degreasers use advanced cleaning fluids that have low surface tension, allowing them to penetrate tight spaces and remove contaminants without leaving any residue. This precision is crucial for medical device manufacturers, where even trace contaminants can compromise device performance and patient safety. Additionally, vapor degreasing runs as a closed-loop system, which minimizes environmental impact by recycling the cleaning fluid for reuse. This makes it not only highly effective but also more sustainable than many alternative cleaning methods.
Ultrasonics enhances vapor degreasing by using high-frequency sound waves to dislodge contaminants from surfaces. This technique is particularly effective for removing particulates from complex geometries and tight spaces common in medical device electronics. Validation protocols must establish optimal frequency, power settings, and exposure time to ensure effective cleaning without damaging components.
The consistency and reliability of vapor degreasing make it particularly suitable for process validation. In the highly regulated medical device industry, where compliance is critical, vapor degreasing ensures that cleaning procedures are reproducible and meet regulatory requirements. By validating the vapor degreasing process, manufacturers can guarantee that each part is thoroughly cleaned to the highest standards, which is essential for device performance, safety, and overall quality assurance.
Other cleaning methods:
-Aqueous cleaning systems use water-based detergents to remove contaminants. These systems can effectively clean water-soluble contaminants but require thorough drying procedures to prevent moisture damage. When used with ultrasonic assistance, aqueous systems can enhance cleaning effectiveness, especially for less complex assemblies. However, proper validation is essential to ensure water quality, detergent concentration, and drying protocols are strictly controlled.
-Selective, or spot cleaning techniques focus on specific areas of electronic assemblies, enabling manufacturers to target critical regions while protecting sensitive components. These manual cleaning methods require precise application and careful validation to ensure that protected areas stay uncontaminated by cleaning agents or displaced contaminants.
Validation methodology
Process validation for electronics cleaning follows the established Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) methodology, providing a systematic approach to ensuring cleaning effectiveness.
Installation Qualification focuses on showing that cleaning equipment is installed and configured according to the manufacturer’s specifications. This includes verification of utilities, safety systems, and equipment calibration. For vapor degreasing systems, this might involve verifying temperature controls and solvent recovery systems. For aqueous cleaning systems, IQ would confirm proper water quality, filtration systems, and drainage.
Operational Qualification shows that the cleaning process performs as intended across its operational range. This involves testing the process under different conditions to show acceptable operating parameters. For electronics cleaning, OQ typically includes finding optimal cleaning agent concentrations, setting up proper temperature ranges, defining cycle times, and verifying rinsing and drying effectiveness.
Performance Qualification provides evidence that the cleaning process consistently produces acceptable results under actual production conditions. This stage involves cleaning multiple batches of production components and testing them against established cleanliness criteria. Manufacturers must prove process consistency across different production runs, operators, and part variations.
This three-stage validation approach provides diagnostic and medical device manufacturers with a structured framework for systematically evaluating cleaning effectiveness and ensuring that the process consistently produces electronics that meet cleanliness specifications.
Best practices for cleaning validation
Effective electronics cleaning validation begins with aligning process controls to device function, reliability expectations, and regulatory demands. To ensure consistent performance and full regulatory traceability, manufacturers should consider the following:
–Define measurable acceptance criteria. Establish cleanliness limits that reflect the specific functional and risk profile of the device, for example, maximum ionic residue levels or particulate limits that could interfere with diagnostic accuracy or device longevity.
–Select appropriate test methods. Choose analytical techniques suited to the types of contaminants identified as risks. These may include ion chromatography, surface insulation resistance testing, and visual inspection.
-Establish and control process parameters. Define critical cleaning variables such as fluid type and concentration, temperature, exposure time, and drying method. Real-time monitoring of these parameters helps ensure consistency and repeatability.
-Document procedures comprehensively. Maintain current Standard Operating Procedures, validated cleaning parameters, maintenance records, and training logs. This documentation supports audit readiness and regulatory compliance under standards such as those stipulated by FDA and ISO.
–Verify material compatibility. Ensure that cleaning agents do not degrade sensitive components or materials. Compatibility testing should be incorporated early in the validation process.
-Monitor and verify ongoing process control. In addition to statistical process control and equipment verification, conduct periodic cleanliness testing to confirm that results stay within validated limits over time.
Establishing protocols
While establishing robust electronics cleaning validation requires investment from medical device manufacturers, the returns justify the expenditure in several ways:
-Reduced field failures translate directly to lower warranty costs and fewer product returns. Meticulously cleaned electronics experience fewer contamination-related failures, improving device reliability and ultimately contributing to patient safety by ensuring devices perform as expected in critical applications.
-Streamlined regulatory submissions benefit from well-documented cleaning validation, which can reduce questions from reviewers and accelerate time to market. Regulatory bodies increasingly scrutinize cleaning validation as awareness of its importance grows.
-Lower product liability exposure results from proving thorough cleaning validation, which can help mitigate liability concerns in case of device failures by showing adherence to industry best practices and regulatory requirements helping ensure patient safety.
-Manufacturing efficiency improvements often accompany validated cleaning processes, which typically improve first-pass yields and reduce rework requirements. The consistency and reliability of validated processes translate to more efficient operations and lower production costs, contributing to overall product quality.
Futureproof validation
Electronics cleaning validation is a critical element in ensuring medical device and diagnostic equipment quality, regulatory compliance, and patient safety. As next-generation devices continue to incorporate more complex and miniaturized electronic components, the importance of validated cleaning processes will only increase.
By implementing structured validation programs that show consistent cleaning effectiveness, medical device manufacturers can enhance reliability while meeting stringent regulatory requirements. In the highly regulated medical device environment, this investment in quality processes provides both regulatory compliance and a competitive advantage through improved product performance and reliability.
source:mddionline
https://www.mddionline.com/components/electronics-cleaning-validation-ensuring-quality-safety-in-medical-device-manufacturing
This article is for knowledge dissemination only. If it involves infringement, please contact us for deletion.









