UDI at 10: Moving into The Future
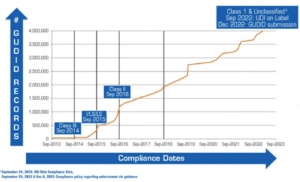
Fig. 1 – Number of devices recorded in the FDA Global Unique Device Identification Database (GUDID). Source: U.S. Food & Drug Administration
September 2023 marked the 10-year anniversary of the day the FDA’s Unique Device Identification (UDI) requirement first took effect. In that time, UDI went from an idea to a framework to a law; its GUDID database now uniquely identifies and holds data on more than 4 million medical devices and is the foundation for thousands of daily lookups and transactions.
UDI’s progress is exceptional, considering where it started, and the many diverse stakeholders involved. Yet many professionals that were most responsible for creating and advancing the UDI system are not celebrating these accomplishments but instead are focused on moving the program forward. This article presents a snapshot of the current state of the UDI and how the movement may evolve.
THE ENGINE IS BUILT; WHO WILL DRIVE?
The foundation for multiple UDI use cases and benefits has been set. The FDA set a phased implementation schedule for manufacturers to create and register unique device identifiers for most non-Class 1 medical devices, and the final mandatory participation deadline was December 2022. As of August 2023, there were 4.15 million unique device records in the GUDID database. The database first surpassed 1 million entries in September 2016, reached 2 million in 2018, 3 million in 2021, and 4 million earlier in 2023.
In June 2023, there were 2,367 file downloads from the online database, which averaged 6,221 user sessions per day, about a third of which came from outside the United States. In 2015, the U.S. Department of Health and Human Services issued a rule requiring electronic health record (EHR) systems (e.g., Allscripts, Cerner, Epic, and others) to support UDI in their software for it to be certified. Besides requiring medical devices to be uniquely identified and registered in GUDID, the FDA requires a GUDID reference on other data submitted to the agency. The FDA reported that 89 percent of the device recall notices it received in Q3 2023 included UDI data, double the level from Q1 2022 (see Figure 1).
The entities that have had to put the most into the UDI program — device makers and the FDA — each want to see it used for more than basic compliance. They have invested a lot to create the system and want it to produce more benefits in patient safety, hospital reimbursement, supply chain efficiency, and security and clinical research. There is a sense that many stakeholders are waiting for others to make the next move. Until something happens (for device makers, UDI marking and registration were mandatory, not voluntary), many organizations will continue to use their own data systems and related processes.
UDI advocates are frustrated by the lack of adoption momentum, particularly in the provider sector because many UDI-based use cases have proven their value. For example, the NEST Coordinating Center (cc) Playbook for Health System UDI Implementation at the Point of Care that was published in 2023 documents different ways providers can benefit from UDI and references real-world examples. 1 Every source interviewed for this article could cite numerous successful programs.
WHAT’S NEXT?
Increased adoption is expected to take three forms: incremental growth from medical device makers and distributors as they rationalize their systems and replace NHRC, NDC, and proprietary number systems used on products, packaging and internal processes; internationally, because the UDI program has spawned similar efforts in the EU and more than a dozen other countries; and the U.S. healthcare sector, where hospital utilization is seen as the Holy Grail for UDI to produce patient safety benefits.
AIM and the American Hospital Association’s Association for Health Care Resource & Materials Management (AHRMM) are currently among the organizations that are most actively promoting UDI adoption by educating potential users. AHRMM scheduled its 2023 UDI Forum for approximately one month after AIM held its own. AIM is active in AHRMM’s UDI Learning Community, which has produced many resources to help hospital professionals to use the UDI system and see its value.
Many professionals in the UDI community believe incremental adoption will continue but there won’t be a significant update without a new mandate. The mandate is not likely to come from the FDA, which has fulfilled its mission of creating the UDI system.
Hopes for a regulatory catalyst suffered a setback in the summer of 2023 when the National Committee on Vital and Health (NCVH) Statistics recommended against requiring including the Data Identifier (DI) segment of the UDI (the UDI-DI) on the standard 837 electronic claims forms for insurance claims for procedures involving implants. Doing so would help ensure that specific devices are associated with specific patients. That would be an integral step to improving recalls, making data available for robust postmarket surveillance to support patient safety, and it could help streamline reimbursement operations.
Natalia Wilson, MD, MPH, called the NCVH claims form decision “very disappointing.” Wilson is executive director of the Center for Healthcare Delivery and Policy at Arizona State University and co-authored the NESTcc UDI implementation playbook for health systems.
The requirement for including the UDI on claims forms is considered stalled, not dead, because it has many supporters. “If that were to go forward, I think that will be a huge driver for UDI adoption,” says Konduri of the FDA. “We’re confident it will go through. The issue is when.”
Several weeks before NCVH opted not to recommend supporting UDI on claims forms, the American Medical Association published an editorial that endorsed the proposal and touted the benefits it could produce. Here is an excerpt from the Journal of the American Medical Association (JAMA) article: “Surveillance of all medical devices, including those modified through PMA supplements, would become feasible, enabling safety concerns to be prospectively identified. More timely notification could also be provided to patients who had been treated with a recalled device, thereby mitigating harm….American healthcare is built to facilitate transactions, and the system’s current transaction standards don’t enable tracking of UDIs. This means that the over 3.5 million devices with UDIs today are functionally invisible to the patients that use them, the providers that purchase them, and the payers that reimburse for them.”2
So, absent an imminent implementation deadline or other regulatory catalyst, expect continued UDI expansion in fits and starts. Many UDI professionals are not satisfied with the current situation, but they don’t think it is permanent either.
“I feel we’re closer to getting there, but we’re not getting there,” says Wilson.
“I think we’re going to overcome the inertia,” says Capatch of Geisinger. “There is so much opportunity; that is what continues to drive us.”
REFERENCES
- “A Playbook for Health System Unique Device Identifier Implementation at the Point of Care,” Nestcc, 2023.
- Hope Caughron, et al., “Medical Device Modifications Through Premarket Approval Supplements—Ensuring Patient Safety,” JAMA, April 12, 2023.
Article Source: MEDICAL DESIGN BRIEFS









