As IUD demand surges, CooperSurgical designs new Paragard placement system
CooperSurgical has launched a new system for easier insertion of its Paragard intrauterine device as more women look to IUDs following abortion restrictions and threats to birth control in the U.S.
An intrauterine device (IUD) is a small, reversible device placed in the uterus to provide birth control.
CooperSurgical said it recently won FDA approval for its new single-hand inserter, saying it makes the Paragard copper IUD “an even more accessible contraceptive choice for both providers and patients.”
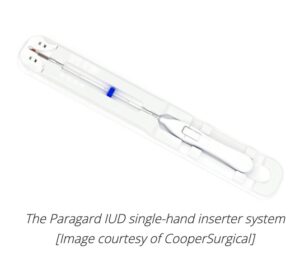
In a news release, Dr. Lee Shulman, a board-certified obstetrician-gynecologist and professor at Northwestern University’s Feinberg School of Medicine, described the single-hand inserter as an “intuitive” device that “simplifies the insertion process, making it convenient for healthcare providers and their patients.”
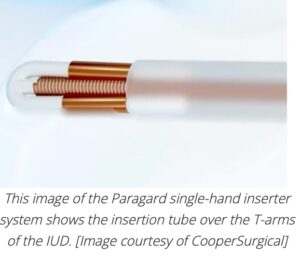
CooperSurgical designed the insertion tube with a built-in loading tip, which uses the device’s packaging tray to fold the T-arms of the Paragard IUD against its stem. Then, the physician slides a button forward to advance the insertion tube over the tips of the T-arms.
The insertion tube also has an adjustable blue flange to mark the appropriate uterine cavity depth (as measured before placement using a uterine sound).
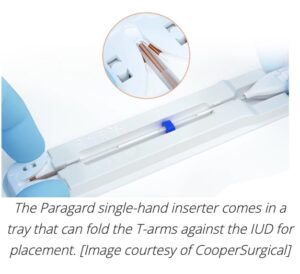
When the insertion tube is inside the patient at the correct depth for placement, that same button slides back to release the T-arms in the uterine fundus.
All this, CooperSurgical said, “simplifies the placement procedure without compromising the reliability of Paragard itself.”
CooperSurgical parent company, Cooper Cos., is the world’s 35th-largest device manufacturer, according to Medical Design & Outsourcing‘s 2024 Medtech Big 100 ranking by revenue.
Growing demand for IUDs
The prescription-only Paragard IUD is immediately reversible and provides more than 99% efficacy for up to a decade, CooperSurgical said.
Paragard is the only non-hormonal IUD contraceptive with FDA approval (which dates back to 1984), though other non-hormonal IUDs are the subject of ongoing research or actively under development.
The Paragard’s coiled, copper wire prevents pregnancy by releasing copper ions that keep sperm from fertilizing eggs. The latest design update for easier placement could make the IUD more competitive against other methods of birth control as demand for contraception climbs more broadly.
“We are constantly striving to meet the evolving needs of healthcare providers, and the patients they serve,” CooperSurgical President Holly Sheffield said in her company’s news release. “With nearly 8 million women choosing Paragard, the new inserter simplifies the placement process and marks a significant milestone for the product. It reaffirms our mission to deliver innovative and safe solutions that positively impact the lives of patients and providers everywhere.”

Women increasingly sought access to long-term, reversible birth control methods like IUDs after former President Donald Trump’s election in 2016. While campaigning, he pledged to reverse the Affordable Care Act (ACA), which requires coverage of FDA-approved contraception.
In 2022, the U.S. Supreme Court (including three justices appointed by Trump) ended the constitutional right to abortion by overturning Roe v. Wade. Planned Parenthood reported a 41% increase in IUD appointments in the weeks following that ruling.
Since then, Republican lawmakers in several states have threatened to restrict access to contraceptives and Trump — campaigning again this year — said he was open to the idea before backtracking. Meanwhile, scores of former Trump Administration staffers have contributed to a post-election playbook that includes reversing FDA approval of mifepristone and barring ACA coverage mandates for the morning-after pill and men’s contraceptives.
article source: Medical Design & outsourcing









