5 tips for meeting new device packaging regulations before it’s too late
The European Union Packaging and Packaging Waste Regulation (PPWR) is advancing rapidly through the legislative process, aiming to address the EU’s approach to packaging waste and shift to a circular economy.

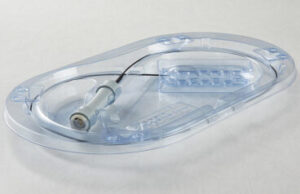
Plastic Ingenuity’s recyclable and optimized PET pipette packaging for Beckman Coulter Diagnostics [Photo courtesy of Plastic Ingenuity]
This regulation is a critical component of the EU Green Deal, which seeks to cut greenhouse gas emissions by at least 55% by 2030 and achieve net-zero emissions by 2050.
Understanding and complying with these new rules is critical for medical device manufacturers, as noncompliance could result in significant fines or even removal from the market.
With changes on the horizon, now is the time to begin preparations.
1. Follow the lead of other industries.
The food and beverage sector has a head start in sustainable packaging design and the medical device industry can learn from this experience. Resources such as the Association of Plastic Recyclers (APR) Design Guide and RecyClass Design for Recycling Guidelines provide valuable insights into designing for recyclability for the consumer market.
Simple changes such as reducing label size, transitioning to polyolefin labels, or removing detrimental additives or colors can make a drastic difference in the recyclability of a package. By adopting and adapting these proven methods, medical device manufacturers can enhance packaging sustainability while complying with upcoming regulations.
2. Start with recyclable materials.
Medical device packaging often involves materials essential for product sterility and safety but are not readily recyclable.
Plastic Ingenuity thermoformed PETG packaging with Tyvek lid, a standard sterile barrier system for medical devices. [Photo courtesy of Plastic Ingenuity]
For instance, PETG (polyethylene terephthalate glycol) is a common material, and with a lower melting point than PET, PETG can contaminate the recycling stream. One innovative development is transitioning from PETG to APET (Amorphous PET) materials. Specific grades of APET can withstand sterilization while being recyclable within the PET stream. Proper testing and documentation will be crucial to prove the recyclability and compliance of APET under the new regulations.
By Jan. 1, 2028, the European Commission will establish design for recycling and recycling performance grades for packaging. From Jan. 1, 2030, packaging shall comply with the design for recycling criteria. While contact-sensitive medical device packaging is exempt from this 2030 deadline, exemption will be reevaluated in 2035.
Resources like the Healthcare Plastics Recycling Council’s Design Guidance can aid medical device packaging professionals in making informed material choices that align with current and anticipated recyclability regulations.
3. Incorporate recycled content.
Recycled content plays a crucial role in promoting a circular economy, decreasing our dependence on fossil fuel-derived plastic, and reducing the carbon footprint of packaging.
The PPWR sets ambitious targets for incorporating recycled content into various types of packaging:
By 2030:
- 30% for contact-sensitive packaging made from PET
- 10% for contact-sensitive packaging from other plastic materials (excluding single-use beverage bottles)
- 30% for single-use plastic beverage bottles
- 35% for all other plastic packaging
By 2040:
- 50% for contact-sensitive packaging made from PET
- 25% for contact-sensitive packaging made from other plastic materials
- 65% for single-use plastic beverage bottles and other plastic packaging not covered by the previous points
The medtech industry must develop pathways to incorporate recycled content into sterile barrier systems (SBS). ISO 11607 (Packaging for Terminally Sterilized Medical Devices), which requires full traceability of SBS packaging materials, currently excludes mechanically recycled content as a possible feedstock. However, using advanced recycled materials will help manufacturers meet future recycled content requirements while complying with this ISO standard.
Since advanced recycled materials break down to their monomer level, their performance and integrity are equivalent to virgin fossil fuel-derived feedstocks. Compliance with ISO 11607 is possible through the mass balance approach to certify the recycled content and maintain traceability. Verifiable bookkeeping distinctly tracks and documents the use of recycled versus virgin materials throughout the supply chain, ensuring transparency and traceability of the amount of recycled content to be claimed.
4. Upgrade your testing and validation process.
As regulations evolve, integrating sustainability into package testing and validation processes becomes increasingly important. This includes:

Plastic Ingenuity team members inspecting thermoform parts in an ISO Class 8 clean room. [Photo courtesy of Plastic Ingenuity]
- Empty space ratio and package minimization: Even for contact-sensitive medical device packaging, the requirement for packaging minimization is in scope and must be documented. The ratio of empty space in relation to the packaged product(s) shall not exceed 40%. Components unnecessary for performance shall be removed, and weight and volume shall be decreased to a minimum to ensure functionality.
- Failure point analysis: Identify the minimum material necessary to maintain package integrity to reduce the packaging footprint.
- Life cycle assessments (LCAs): Perform LCAs to evaluate the environmental impact of your packaging and identify opportunities to reduce material usage without compromising performance.
- Evaluation of recyclable materials: Test new recyclable materials within your verification protocols to ensure they meet regulatory requirements and performance standards.
Take advantage of reverification and revalidation efforts with regulators by incorporating package redesign into V&V procedures.
5. Stay tuned for labeling requirements.
Proper labeling for disposal by the end consumer will be essential for compliance with the PPWR.
From 42 months after the regulation’s enforcement, packaging must include a label with information on its material composition. Harmonized logos and formats for these labels will be provided 18 months post-entry into force.
Stay up to date on these requirements and adapt your labeling practices as more guidance becomes available.
Article source: Medical Design & Outsourcing









