Can Design Controls Accelerate Medical Innovation?
We are living in an unprecedented era of rapid medical innovation in the face of COVID-19. The speed of development for devices and diagnostics has been driven by the urgency of the pandemic resulting in enhanced public-private collaboration, access to funding, and a reduced regulatory burden.
While the pace of medical innovation today can be seen as an inspiring silver lining, it also highlights the lack of that speed over the past 20 years. When the dust settles and COVID-19 is behind us (and it will be behind us), I find myself asking the following questions:
• What lessons will we have learned to keep the positive momentum going into the future?
• What will happen when our public-private collaborations, access to funding, and regulatory pathways to commercialization resume to the status quo?
• Will the pace of medical innovation also wind back down to the pre-COVID era?
To the last of those questions, I say, “No.” There’s an infinite number of lessons to be learned from navigating today that we can apply in the future even without economic stimulus and EUAs.
And with that, I ask the question for which this post is named:
• “Can design controls accelerate medical innovation?”
While this question may seem out of place, there is logic to it. This logic is based on a 20-year observation brought to into focus with 2020 vision.
Innovation begins at the fuzzy front end of design, so that is where this journey begins. From this vantage point we can see significant hurdles. They are in front of us, and we clearly see them. We know that one day, down the road, we’ll need to produce our solutions under good manufacturing practices. We will have to do verification and validation testing to get there. We have a complex ecosystem to navigate with barriers to entry including market considerations, intellectual property, regulatory pathway to approval, and medical economics.
Today, though, on this journey of medical innovation, our biggest concern may likely be focused on science and technology challenges. Our priority is product development.
But there is something that might delay our time to market. It is something that could even cause our invention to fail in the market. That thing is design history. It is out of focus, unclear, and in the rearview mirror. There could be something in our blind spot, left behind years ago, that is going to wreck us.
Flashback to the mid 1980s. There was a study finding 44% of voluntary recalls from medical devices with quality problems in the market could have been prevented in design. This study was announced by FDA on Tuesday, May 22, 1990, on page 21108, Vol. 55, No. 99 of the Federal Register. That FDA notice read like this:
“The Food and Drug Administration (FDA) is announcing the availability of a report in the compliance guidance series entitled ‘Device Recalls: A Study of Quality Problems.’ The report provides an analysis of device quality problems that led to recalls from fiscal year 1983 to 1988. This information should be useful to medical device manufacturers in identifying those areas where controls should be emphasized to prevent or minimize quality problems that could lead to production and distribution of defective medical devices.”
Here, FDA is communicating that this study may be useful to companies. My interpretation is that they are saying, ‘Hey, if you control your design process, you can increase your product quality and minimize costly failures in the field.’
Seven years after the notice in the Federal Register, FDA published “Design Control Guidance for Medical Device Manufacturers.” This guidance document was never intended to constrain design but rather to encourage companies to develop a controlled process. That is, a process that is repeatable. After all, any quality process is one that is repeatable. In my opinion, this document was written with good intentions.
 Above: Cover page of FDA’s “Design Control Guidance for Medical Device Manufacturers.”
Above: Cover page of FDA’s “Design Control Guidance for Medical Device Manufacturers.”
Some parts of a process are so critical to success, it is essential to control them. We might agree that in manufacturing, process controls can be critical. Consider injection molding. I would argue that controlling the heat, pressure, flow, and cooling are all critical.
I am a champion for human-centered design. For me, an FDA publication on design controls is a good thing. Such a publication is emphasizing that design is so critical to the success of outcomes, we should consider making design a repeatable process.
Today we are living in a different era. In 1997 when FDA published this guidance document, it was written for medical device manufacturing companies. These companies owned the process from user needs to medical devices. Today, however, the world looks different. Clinical immersion and medical innovation programs are abundant at universities and hospitals all around the world. A significant amount of design work is happening in research labs and clinics. Yet there is no guidance document for medical innovators.
I’ve spent my career working among students, faculty, and clinicians who are the champions of new medical innovations. The vast majority of them have never heard of design controls. When user needs, design inputs, and the design process are all happening in hospitals and universities, while design outputs and the medical device are owned by medical device manufacturers, there’s a significant disconnect.
FDA’s guidance document on design controls has been a double-edged sword. One side of the blade is our own misinterpretation of the Waterfall Design Process (shown below) as a stage-gate. The other edge is transfer of design from where it started (often in academia, hospitals, and R&D teams) to manufacturing. Both edges of the sword create a reality of reverse engineering design controls.
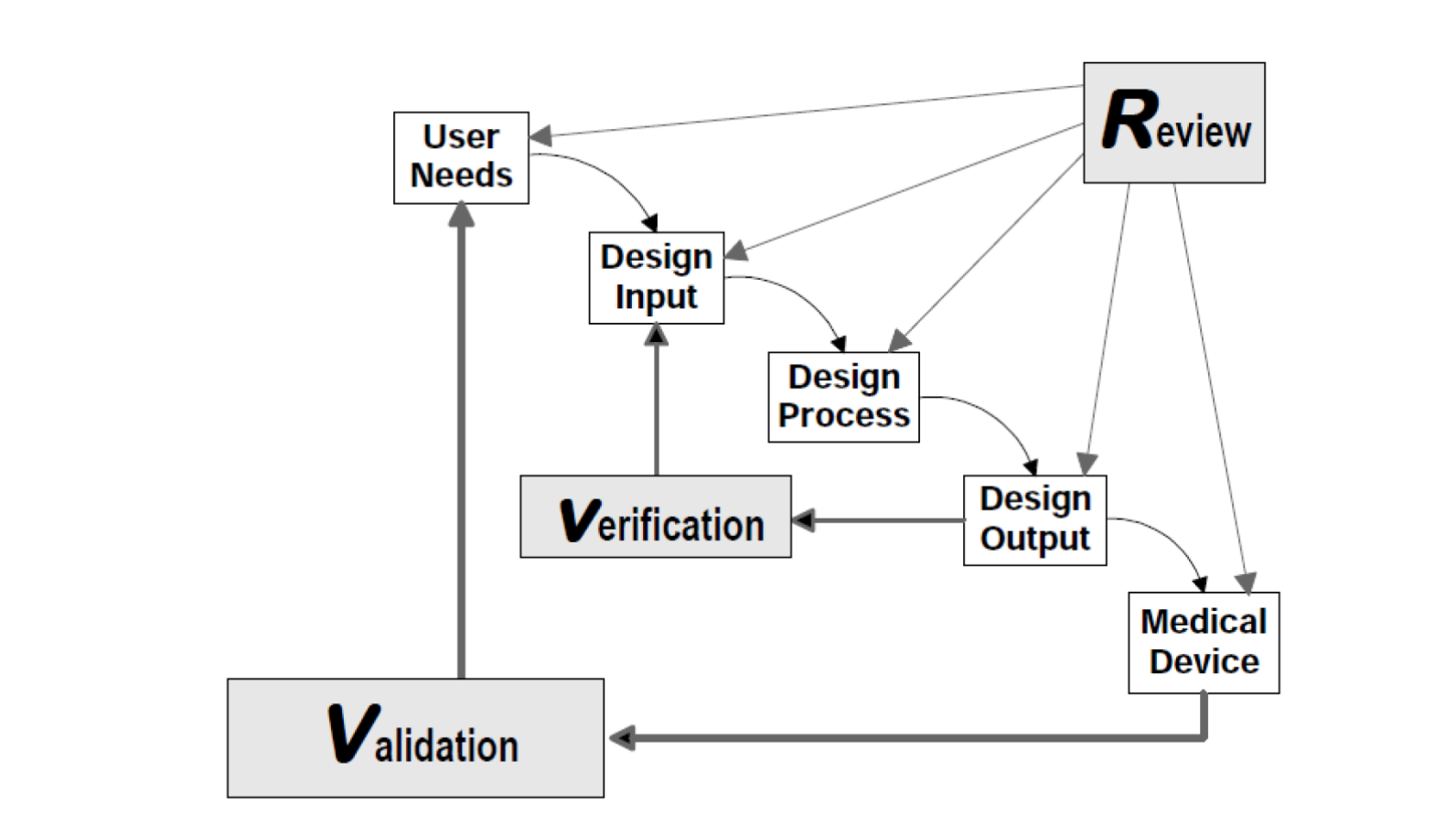 © All rights reserved. Application of Design Controls to Waterfall Design Process. Health Canada. Adapted and reproduced with permission from the Minister of Health, 2019.
© All rights reserved. Application of Design Controls to Waterfall Design Process. Health Canada. Adapted and reproduced with permission from the Minister of Health, 2019.
What do I mean by misinterpretation of the Waterfall? FDA never intended for this to be a stage-gate process. A close look at the figure (shown above) from the guidance document shows an iterative loop. If the figure is not clear enough on its own, the authors went on to reiterate the point in the paragraph just below the figure, where they state: “In practice, feedback paths would be required between each phase of the process and previous phases, representing the iterative nature of product development.”
This sharp edge of the blade works in concert with the other half of the double-edged sword creating a time lag between exploring unmet needs and delivering final solutions. The misinterpretation also creates a lost incentive for staying connected to the end-user throughout the lifecycle of design. This is only exacerbated when design is being performed by academic and clinical innovators. Not only do we have separation in time, but a likely added separation of institutional knowledge between the design effort and the team responsible for commercialization.
The bottom line is this: When we don’t begin recording design history and formalizing a traceability matrix until after we have a single known solution, we are inherently not doing design control. Rather, we are performing an administrative task to fulfill a design control requirement. Worse than the time lost and resources required to build design history is the lack of benefit from a controlled design process. Controlling the process leads to better outcomes in the marketplace. An administrative task to backfill history provides no such commercial advantage.
Is it possible to do formal design controls with a human-centered iterative approach? Yes. FDA lets us know that in its own guidance document. It is up to us to embrace, document, and deploy an evolutionary process in our organizations. More important is the connection to where design is happening, at universities and academic institutions. If we can incorporate a process to capture early design history in these settings, we can improve technology transfer and ultimately accelerate and improve downstream success of innovative products in the market.









