Additive Manufacturing: Creating the Next Generation of Orthopedic Implants
Additive Manufacturing (AM) is an exciting technology being explored for use in the medical device landscape. From improved patient outcomes to point-of-care manufacturing, 3D printing offers a host of benefits that promise to transform the orthopedic implant industry once fully implemented. For medical companies to leverage this novel manufacturing technology, extensive materials testing will be needed in order to validate new processes and prove that their products are functionally comparable (or superior to) those made with traditional processes. Adding to the complexity, this application currently exists in a dynamic regulatory environment where the industry is still debating best practices. Companies seeking to explore this space will need to implement a highly adaptable materials testing program in order to realize the full potential of additively manufactured orthopedic implants.
DISADVANTAGES OF TRADITIONAL MANUFACTURING
The geometry of anatomical structures is exceedingly complex, and traditional subtractive manufacturing is severely limited in the geometries it can produce, as well as the number of pieces of equipment required to make an implant. Furthermore, the time required for machining can be significant, especially when working with harder metals such as titanium, which is frequently used for orthopedic implants due to its mechanical properties and biocompatibility. Technological improvements in traditional manufacturing processes such as computer numerical control (CNC) machining remove operator error and allow for a wider range of geometries with a single machine, but still fail to address the medical shortcomings of traditionally produced implants.
Casting requires appropriate tooling and jigs to pour molten materials into — a process that can produce more complex geometries but is often cost prohibitive due to the capital required for the tooling and jigs. For this reason, many implants are only available in discrete sizes that are based on historical patient data representing the average groupings of patient anatomical geometry. This sizing model is detrimental to patient outcomes because physicians often need to remove more bone to ensure a proper fit. A study performed using finite element analysis (FEA) and the comparison of traditionally and custom designed AM implants found that the amount of bone removal could be reduced by 40 percent when using custom-sized implants. 1
Although orthopedic implants are often intended to be in use for 30+ years, poorly sized implants can result in unexpected stress concentrations from daily wear and tear, reducing the total life of the implant. The revisional surgery required to remove and replace damaged or broken implants is costly and presents a much greater risk to the patient than the original surgery. A study of hip replacements shows only just over 80 percent of implants will last longer than 25 years, a number that could potentially increase with AM produced implants. 2
UNIQUE BENEFITS OF ADDITIVE MANUFACTURING
One of the primary concerns for any implant is whether it will be accepted by the body. AM techniques allow for the inclusion of porous structures within the implant, a crucial feature that allows for the biomedical fusion of the implant.
Manipulation of implant porosity also allows for weight reduction of the part, helping it to better match actual bone density. Research has shown that porosity can be manipulated to alter the mechanical properties of the implant. 1For example, a spinal implant should flex and compress a similar amount to the adjacent spinal discs to avoid introducing excessive pressure and subsequent pain for the patient. Adjusting the porosity allows the implant to match the mechanical stiffness of the natural surrounding bone. These design improvements all translate to improved outcomes for the patient, minimally invasive surgeries, and reduced possibility of implant failure down the road.

A 3D printed dog bone shaped test specimen, also known as a coupon. (Credit: Instron)
Outside of the realm of patient care, another unexpected benefit of additive manufacturing is its ability to completely upend the existing supply chain and distribution of biomedical implants. Especially today, as supply chains are increasingly susceptible to material shortages and the costs of transportation are skyrocketing, the opportunity to mitigate these risks is highly desirable within the industry. AM manufacturing requires less material input than traditional manufacturing and produces much less material waste, reducing the supply chain burden. The overall process for AM also requires less space and fewer steps to produce a finished product, meaning a more localized distribution network can be utilized. This is particularly advantageous for fully customized implants, which could potentially be prototyped and/or produce at the point of care, eliminating non-value-added steps of the supply chain. The proximity of the production to the point of care also enables greater physician feedback into the production process, making them a key stakeholder in the supply chain.
PROCESS VALIDATION CHALLENGES
Within the biomedical industry, novel innovations always experience significant regulatory hurdles before being commercialized and widely adopted. Implants are considered class 3 products by the FDA, the most scrutinized class of product due to the impact of potential failure to the patient. Guaranteeing implant safety is further complicated by the long-term usage of the product, requiring premarket approval (PMA) to prove efficacy and safety. AM processes introduce numerous variables to manufacturing (see Table 1).

A universal testing machine can be used to perform a wide range of mechanical tests, including tensile, compression, flexure, torsion, and more. (Credit: Instron)
Process validation requires a complete understanding of how each of these AM process parameters will affect the device quality and performance. Studies are required to ascertain the process repeatability within a given build cycle, across multiple build cycles, and on different printing systems within a facility. Additionally, all the system software will need to undergo proper validation per 21 CFR 820. Despite the effort required to fully validate an AM process, the benefits to both the manufacturer and patient are clear, providing efficiency cost savings and improved efficacy, respectively. These hurdles are actively being tackled by some of the largest implant manufacturers in the industry, as the FDA has only released guidance documents to date. The responsibility is on the manufacturer to provide ample evidence to regulatory bodies that the product is the same or better than what is produced using existing technologies, and mechanical testing is a crucial piece of measuring process performance.

A cobot can be added to a universal testing system, allowing an operator to simply load a specimen rack and the cobot will automatically load and unload the entire batch for testing. (Credit: Instron)
MECHANICAL TESTING CONSIDERATIONS
Traditionally produced implants require extensive mechanical testing including quantification of raw material properties, measurement of device responses to anatomically representative stresses, and determination of implant fatigue performance, among other things. The use of AM creates a need for extensive qualification testing related to the variable parameters mentioned in the previous section. By creating test coupons, a range of static tests can be performed on the material, including tensile, compressive, flexural, and torsional testing. A universal testing system is optimal for performing this kind of qualification, along with the necessary fixturing for each test type. Partnering with mechanical testing experts is important to ensure compliance with relevant ASTM or ISO material standards such as ASTM D638, ASTM D695, ASTM D790, and ISO 6892. Generally, the properties in question will be the material’s stiffness [elastic modulus], yield strength, ultimate strength, and ultimate elongation. System accuracy related to force and displacement is crucial when attempting to determine the cause of variations in material properties between slight modifications of AM process. Ensuring that a supplier can provide materials testing systems with high accuracy load cells and extensometer options is invaluable for determining material properties with the necessary resolution for parameter optimization.
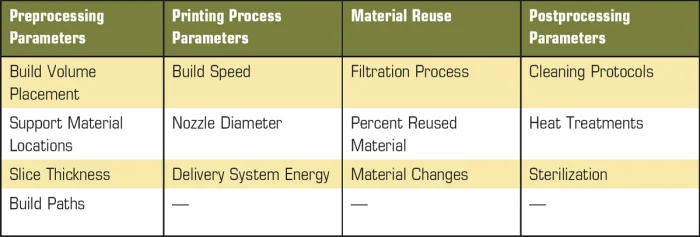
Table 1. AM processes introduce numerous variables to manufacturing.
The use of coupons is not solely limited to the qualification process. Many manufacturers will make the decision to incorporate coupon testing as an in-process control, placing coupons on the print tray between parts or in specific locations of the tray for each build. The coupon placement can be purposefully done to capture the “worst case” scenario for the system, based on the orientation or the location within the build volume. Additional mechanical testing will need to be performed to prove the coupon is representative of the finished part but doing so will make the subsequent in-process testing simpler and more repeatable.
To address the sheer volume of testing that will need to be performed for validation, automation solutions such as a cobot could be beneficial, removing the need for dedicated system operators. The cobot is designed primarily for flexibility and can be quickly taught to pick and place specimens from any arrangement. This flexibility also extends to the various test types, meaning a cobot solution can perform tensile, compressive, or flexural tests with minimal modifications to the software aside from the necessary test method changes. Improving test throughput will be a significant factor in reducing the time to market for the product.
AM has the potential to completely revolutionize the landscape of orthopedic implant production, improving patient outcomes while circumventing many supply chain challenges. Designing and printing implantable prostheses customized to the patient ensures an optimal fit, with as little unnecessary augmentation to the existing skeletal structures as possible. AM techniques can allow implants to be provided at or directly near the point of care, increasing responsiveness to patient demands and easing supply chain burdens. Manufacturers pursuing this technology will need a flexible and adaptable mechanical testing program, ideally partnering with a supplier that is familiar with the unique testing challenges that come along with AM processes.
REFERENCES
Murr, L. (2018). Additive Manufacturing of Biomedical Devices: An Overview. Materials Technology, 57–70.
Shan, L. B. (2014). Total Hip Replacement: A Systematic Review and Meta-Analysis on Midterm Quality of Life. Osteoarthritis and Cartilage, 389–406.
Article source: Medical Design Briefs









