Protein Adhesion and Metal Interaction in Medical Diagnostic Equipment

At a Glance
Preventing protein/biologic adsorption Comparing coatings and stainless steel Test data and results
The medical device market is ever changing and is subject to rapid evolution from a technology standpoint. However, one constant in this fast-paced market is that there are some tricky problems that have plagued research teams and product developers, one of these problems being sticky proteins and biologic materials.
Prevention of non-specific protein adsorption is always a subject of interest leading many device developers to consider surface materials like electropolished stainless steel, MP35N, plastic polymers and other expensive alloys.
This article will examine different surfaces and their ability to withstand chemical instability issues in oxidative environments (e.g. air) and physical degradation (e.g. delamination).
The problem with proteins
While non-specific protein adsorption levels can be less severe of a problem in other industries, in the medical device industry it is a problem to be avoided as much as possible. Modern clinical labs use immunoassay formats that amplify the detection of relevant analyte found in whole blood, serum, plasma, cerebral spinal fluid, urine, and other bodily fluids.
Using immunoglobulins achieves the goal of specific and sensitive capture of the molecules of interest, however, the interreference from non-specific proteins binding to solid surfaces often limits the performance, interfering with the limit of detection due to poor signal to noise ratio.
Setting up the experiment
To find a surface most suited for preventing unwanted protein adsorption, SilcoTek scientists contributed our Dursan coating technology to be compared to 316L stainless steel as well as common competitive fluoropolymer coating, AF1600. The comparison experiment was performed by scientists at Abbott Laboratories.
Quartz crystal microbalance with dissipation monitoring (QCM-D) was used to characterize the anti-biofouling properties of each of the test surfaces. QCM-D monitors changes in oscillation frequency and dissipation of a planar crystal substrate upon adsorption of macromolecules. Sonication was introduced in the test to induce rapid mechanical wear so durability of the coatings can be assessed.
Experimental baseline for the QCM-D measurement was determined by flowing a wash buffer, referred to as WB1, over all sensors for ~ 4 minutes to clean the surfaces and priming them for protein exposure. Protein solution of interest was then flowed over the sensors for 20~25 minutes, followed by another rinse using WB1 for 25 minutes. A minimum of two distinct measurements were carried out for each protein-surface system. A change in the oscillation frequency (Δf) of the QCM-D sensor indicates protein adsorption onto the sensor surface, and Sauerbrey equation (Δm = −C/n·Δf) was used to correlate the adsorbed mass (Δm) to the changes in oscillation frequency (Δf). C is the mass sensitivity constant for the specific sensors used, and n is the overtone used for measurement. In addition, the ratio of change in dissipation to change in frequency can be used to determine the rigidity of the adsorbed protein layer.
Figure 1:
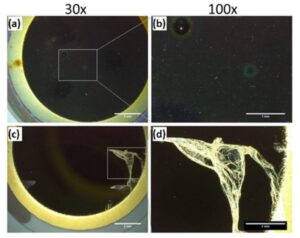
Figure 1: Optical micrograph comparison of Dursan coating (a, b) and AF1600 coating (c, d) after cleaning and sonication treatment.
The best surface for preventing unwanted protein adsorption
Physical Degradation Results: To assess and compare the physical durability of different coatings during use, the authors exposed two types of coatings, Dursan and AF1600, to 10 minutes of sonication in ethanol. Multiple sensors of each coating were sonicated, and all of the Dursan-coated sensors remained intact after the treatment, whereas a few of the sonicated AF1600-coated sensors exhibited coating delamination as shown in Figure 1.
Environmental Performance Results: The protein adsorption properties of Dursan and AF1600-coated sensors after cleaning and sonication were assessed by exposing them to normal human plasma (NHP), which is representative of patient samples utilized in diagnostics analyzers. The authors took care to only use AF1600 sensors that did not show any visible post-sonication delamination in this study. Even so, they observed loss of protein-resistant properties of the treated AF1600 surfaces, as shown in Figure 2, that the frequency and dissipation profiles of treated AF1600 (triangle) did not revert to the baseline levels.
Figure 2:
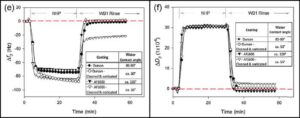
Figure 2: QCM-D frequency (e) and dissipation (f) profiles comparing the adsorption of NHP proteins on untreated (at left) and treated Dursan (circle), at left; and AF1600 (triangle) surfaces.
In comparison, the Dursan surface (circle) exhibited no performance change before and after treatment. The authors suspected that the AF1600 sensors are susceptible to microscopic structural change and deformity when exposed to sonication, so that even without visible delamination, they suffer the loss of the protein-resistance properties.
Conclusion
This experiment is limited to only two surface modification technologies but already shows the impact that proteins can have on performance. As the medical device market continues to evolve, companies will need to move quickly to keep up with the challenges posed by sticky biologic materials and their ability to limit accuracy.
In part, this article is a summary of the study done in partnership between Abbott Laboratories and SilcoTek Corp., titled, “Protein-resistant properties of a chemical vapor deposited alkyl-functional carboxysilane coating characterized using quartz crystal microbalance.” You can read the entire paper here.
Article source:Medical Device and Diagnostic Industry









