3D-printed PEEK-based Spine Implant Cleared by FDA
The implant’s porous structure, enabled by the polymer and Bond3D printing technology, promotes native bone ingrowth, potentially improving patient outcomes.
The first 3D-printed spinal implant fabricated from Invibio Biomaterial Solutions’ PEEK-Optima polymer has received FDA clearance. The PEEK Interbody System is marketed by Nvision Biomedical Technologies, a medical device and implant manufacturer based in San Antonio, TX.
First of its kind
The system consists of cervical and anterior lumbar interbody fusion (ALIF) spine devices. Each element incorporates extensive porous structures that have the potential to promote multi-directional bone ingrowth and improve device fixation. The system reportedly is the first device of its kind made from PEEK-Optima using the proprietary Bond3D additive manufacturing technology that enables printing of solid and porous areas.
PEEK-Optima has already been used in more than 15 million implants. Its mechanical properties resemble those of human bone and it is superior to titanium implants because of its imaging capability, which allows surgeons to more accurately monitor fusion progression, said Invibio.
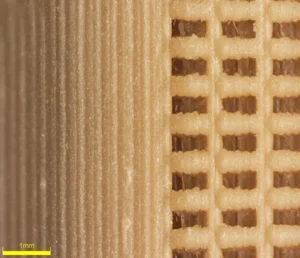
The porous structure of the Nvision interbody system is shown here at 43x magnification. Image courtesy of Invibio Biomaterial Solutions.
The collaboration between Invibio and Nvision dates back several years, particularly in spine and foot-and-ankle devices. In 2020, Nvision received 510(k) clearance from FDA for an osteotomy wedge system made from Invibio’s PEEK-Optima HA Enhanced polymer.
Improved outcomes
The new interbody devices will improve the quality of care and surgical outcomes for patients, according to Dr. Steven Lee, MD, a spine and orthopedic surgeon cited in the press release. “PEEK-Optima’s modulus of elasticity helps to prevent subsidence [sinking of bone next to an implant]. Its radiolucency allows for the confirmation of the fusion process. The benefit of a printed porous structure that mimics native bone allows for bone growth into the device itself, thereby enhancing the construct stability. These combined new implant characteristics of strength, reduced subsidence, boney ingrowth, and radiolucency will provide clinical benefits to all my patients,” said Lee.
Nvision and Invibio collaborated in the development of the 3D-printed PEEK Interbody System, with Invibio carrying out development of the PEEK-Optima and Bond3D technology platform, performance testing early in the process, and eventually filing a new master file (MAF) with FDA to support this and future regulatory submissions.
Nvision and Invibio said they intend to continue expanding the applications of 3D-printed PEEK technology to meet the growing needs of surgeons and patients worldwide.









