Advances in 3D Printing Usher in the Next Wave of Orthopedic Casts

Image Courtesy of ActivArmor
A growing number of adults will need a cast for a broken bone at least once in their lifetime. Fiberglass casts—the most common type used—hold the bone in place, but they come with a host of complications, especially for active adults and children.
That’s why Diana Hall, founder and president of ActivArmor, which develops custom, 3D-printed plastic casts and splints, believes it’s time for an upgrade.
While mentoring kids in her hometown, Pueblo, CO, Hall saw the downsides of fiberglass and plaster casts: children with skin irritation, infection, and scarring because they couldn’t keep their casts clean and dry.
Hall and team developed an alternative that’s breathable, adjustable, 100% recyclable, and can be custom fitted on the spot using 3D printing.
MD+DI spoke with Hall recently to discuss the diverse and growing exoskeleton market, which includes everything from robotic devices to casts. She also shared the reasoning behind ActivArmor’s novel design and its commitment to making the devices affordable for both patients and physicians.
The term exoskeleton is used to describe devices that help people with paralysis stand and walk; devices that support the spine when people are lifting heavy loads; and for splints and casts. How do you define it?
Diana Hall: In the medical device industry, it refers to externally worn protective or stabilizing devices. You can add mechanical functionality so that the elbow or wrist only bends in a certain way, for example. Or you can use it for immobilization, which is what ActivArmor does. We create low-profile, supportive exoskeletons that are custom-fitted to the body.
Market research shows a strong annual growth rate for casts and splints as well as the medical exoskeleton market. Why do you think this is so?
DH: First, we have an aging population. They’re experiencing falls and they’re developing chronic conditions like carpal tunnel that need casts or splints.
Second, people with diabetes need improved technology, like offloading walking boots for foot ulcers. Custom walking boots with bone stimulators can reduce their risk of amputation and even mortality.
Third, exoskeletons can improve healing outcomes and rehab times and are being used more frequently to improve the quality of care and reduce treatment times.
Compliance and complication rates are issues that doctors are looking to improve on. We’ve seen patients chew or cut their cast off because of claustrophobia because it itches or stinks, or because of pain from swelling.
The more 3D printing evolves, the more improvements we’re going to see in the space and the more demand we’re going to see, as well.
What regulatory challenges do 3D-printed casts and splints face?
DH: It depends on what healing outcome claims you’re making with your device. ActivArmor is a Class I medical device. The only claim that we make is that it will immobilize a limb. Our research shows that ActivArmor works at least as well as traditional casts and splints for immobilization for healing. Our devices are ISO 10993-5 certified, and FDA listed. Other devices, such as 3D-printed implantables, or those making Class II claims, will face further regulatory requirements.
ActivArmor devices are adaptable for use with Class II devices, such as ultrasound bone stimulators, to reduce fracture healing times, and muscle stimulators to reduce rehab times. So, our device can improve healing outcomes when used with complimentary Class II devices.
Over the last 10 years, we have successfully fit thousands of patients from hundreds of referring providers. One reason for its safety and efficacy is because you can observe and treat the skin while the patient is healing. If there’s any swelling or post-surgical infection, you can see it and adjust the device immediately and treat the skin throughout healing.
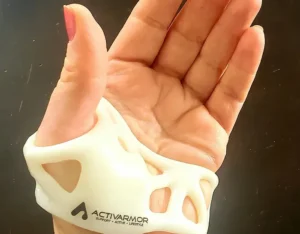
Why did ActivArmor decide to use 3D printing to produce its splints and casts?
DH: It’s not a one-size-fits-all world. Small, medium, and large doesn’t cut it. My wrist looks like an hourglass and my daughter’s wrist looks like a cylinder.
One person might need to be immobilized up to their elbow and another may need support past their elbow or for their ankle up to the knee. If a patient has an incision, a laceration, or surgical hardware, we can custom offset or expose that area.
3D printing allows you to customize the device to an individual’s specific injury and shape right there in the doctor’s office. You can’t do that with a traditional cast. And you don’t have to know CAD to use the design software – it’s a simple web user interface anyone can use. And our custom design software is printer agnostic, so as 3D printing technology gets faster, so will our product delivery.
The products use acrylonitrile butadiene styrene (ABS), a plastic that’s tough but also hygienic. How did you decide on this material?
DH: It’s washable, sanitizable, sterilizable, and non-reactive to skin. It’s ISO certified for biocompatibility, and it doesn’t trap moisture and bacteria against your skin. It has a high melting point so you can get into the hot tub or stay out in the sun, and it won’t deform.
It’s also recyclable, which helps reduce medical waste. The casts themselves are reusable—you can adjust them to use through all healing phases, which cuts down costs.
ABS is also an affordable material, which enables physicians and hospitals with our point-of-care system to keep the cost low for their patients.
What sort of outcomes are you seeing with these casts and splints compared to traditional products?
DH: We’re seeing a reduction in complication rates because you can observe and treat the skin through the cast. You can wash underneath, adjust it for swelling, and tighten it when swelling goes down.
We are seeing an improvement in patient compliance, which improves healing outcomes.
When you combine the cast with a bone stimulator you can now reduce your bone fracture healing time. If you use it with a muscle stimulator you can reduce rehab time, as well.
How does ActivArmor plan to bring its products to underserved communities?
DH: Our devices are covered by Medicaid. We keep the cost to our provider customers at or below Medicaid rates, always.
Doctors can get 3D printers for free with our system. There’s a minimum order—if they order a minimum of eight products per month, they get the entire system for free. If they don’t want to have a minimum order amount, they can purchase the entire system for $9,500. That includes a 3D printer, the materials, the scanning app, all of it.
At the end of the month, we just bill them at 60% of state-published Medicare rates for the devices they’ve ordered. This keeps it affordable for everyone – insurance companies, Medicaid, Medicare patients, and even cash payers.
Any provider, even those that aren’t contracted with us or don’t have a 3D printer, can prescribe one of our devices. They order through an app, and we ship them the device within a couple days.
Where do you see this market—and ActivArmor—heading over the next few years?
DH: Where I see it heading—and where industry experts like Charlie Barocas, the director of the American Society of Orthopedic Professionals, says that he sees it heading—is that traditional casting will likely become less than 20% of the total fracture care market over the next 10 years. Products like ActivArmor are going to take over that majority market share.
We’re already seeing patients advocating for their own health and safety. Patients are going to go where they can get the latest in treatment at the right price. We offer custom logos on all our devices, so patients become walking billboards for their clinic. That’s a marketing opportunity. So not only are clinics able to provide improved healing outcomes and improved patient satisfaction, [but] they also have a competitive advantage and can get external referrals if they’re offering these products.
Looking forward, I see the orthopedic care market as continuing to be very competitive. I see patients becoming stronger advocates for themselves in the healthcare space, and providers offering the latest technology and treatments coming out ahead.
Article source: MDDI









