Multi-Layer Extrusion Processes Tackle Tubing Challenges
In the medical device industry, the trend toward minimally invasive surgical techniques is the driving force for smaller, more innovative medical devices. Devices for vascular and other applications continue to reach deeper into the anatomy with more therapeutic technology, and medical device manufacturers are demanding polymeric tubing that boasts greater precision, tighter tolerances and increased functionality.
As tubing designs become more complex, so does the extrusion technology required to produce them. At the forefront of these advances in medical tubing technology have been developments in multi-layer extrusion technology. Multi-layer extrusion, or co-extrusion, can be defined as the extrusion of multiple layers of material simultaneously to produce multi-layered tubing. Multi-layer technology is primarily employed to improve functionality: to combine a weldable material with one that has certain other performance properties, like lubricity, for example. Such constructions also can increase performance and possibly reduce overall assembly and material costs, making the medical device more cost-effective for the customer.
Other key functionalities may include the addition of active material layers, such as hydrophilic, bioresorbable or drug-eluting layers. The technology and materials now being employed to produce multi-layer products for today’s medical devices has advanced greatly and provides the designer with a wealth of opportunity for optimization of size, materials and functionality.
The use of multi-layer tubing in medical devices allows for:
• The creation of tubing with different properties for the exterior and interior surfaces;
• Materials with different properties to be combined to create unique tubing characteristics;
• Active materials to be located in their optimal location; and
• Bondable materials to be located inside or outside for ease of assembly of complex medical device.
Multi-Layer Extrusion Equipment
Historically, the manufacturing of multi-layer tubing consisted of technicians cobbling together an assortment of various extruders and downstream equipment that were borrowed from other extrusion lines within the company. With this inferior method, it was highly probable that the various extruders all were in the same plane, resulting in longer flow channels, increased pressure and increased residence time of the material.
Frequently, the required layer ratios of the tubing would not match the optimal output of the extruders, which could result in screw speeds being too high or too low. Tubing with inferior mechanical properties could be created from shear degradation or high material residence time. Compounding all of these issues were myriad operator interfaces on various extruders, sizing tanks and haul-off that the operator would have to monitor throughout the production run. The outcome often would be poor tubing performance characteristics and process-control issues. As a result of this, in some cases, successful development runs conducted by engineers or highly skilled technicians could not be transferred to production.
Modern multi-layer extrusion processing equipment is much more likely to be a fully integrated line, with a single operator interface to monitor, control and record the entire extrusion system in real time. The extruder for each polymer layer is selected to deliver the required output speed ranges that do not compromise the mechanical properties of the material. They are positioned to minimize material residence time and generally one or more of the extruders may be mounted vertically or at a 45º angle. Often, one or more of the extruders in the multi-layer system will be a micro-extruder with outputs as low as 50 grams per hour, allowing individual layers to be only a few microns thick. Manufacturers of these micro extruders use innovative extruder throat designs to ensure standard plastic pellets carry forward well in the feed section of the extruder, with no loss of stability.

(Above) Offline measurement of microbore multi-layer tubing at 300X magnification. Photo courtesy of Vention Medical.
Today’s systems also have in-line measurement systems, monitoring not only the outer diameter but also the wall thickness and inner diameter, using refraction of infrared light technology or ultra-sonic technology. Often, these systems provide closed loop control to the extruders, puller and pneumatic or vacuum sizing, to ensure the most accurate dimensional control is maintained throughout the extrusion run. The use of such statistical process control (SPC) systems is driven by customer expectations and improves the ability to meet the increasing demands of the regulatory authorities such as the U.S. Food and Drug Administration (FDA). However, accurate in-line measurement of the thinnest layers continues to pose challenges. Currently, multi-layer manufacturers must use offline measurement of discrete samples using high magnification video measurement systems with magnification up to 350 times.
Materials
When creating multi-layer tubing for a particular application, it is fortunate that many thermoplastic materials can be co-extruded. The most common materials employed are those that traditionally have been used to make medical tubing (e.g., polyamide, thermoplastic elastomer, polyurethane, polyvinyl chloride and polyolefins). These materials can be customized to include drug-eluting ingredients, radiopacifiers, fillers and/or colors. However, the designer must take into account the material’s processing temperature, flow characteristics and melt viscosity.
For example, it would not be possible to co-extrude a high-melt-temperature, high-viscosity material such as polyether-ether-ketone, or PEEK, which has a melt temperature of approximately 660ºF with a material like polyethylene, which has a melt temperature of approximately 260ºF because the temperature differential is too great. Compatible materials will bond when extruded together. In order to combine two chemically dissimilar materials, a tie layer must be used. This provides no additional mechanical functionalities and simply bonds the outer and inner layers.
Multi-layer extrusion technology is particularly suited to the extrusion of expensive novel or active materials that have been developed for a specific function, in that it allows these materials to be located appropriately. Common examples are when a drug-eluting material needs to be on the outer layer or a highly lubricious material needs to be placed on the inner layer. Since multi-layer tubing allows for thin layers of these materials to be placed where they are most effective there can be favorable cost implications.
Common Uses for Multi-Layer Tubing
Multi-layer extrusion tubing products can offer additional functionality through multiple material combinations and can facilitate, reduce or even eliminate secondary assembly processes. The most successful component designs involve the medical device designers working in partnership, from the early stages of the design phase with the extrusion experts who have the necessary knowledge in materials and processing. The following are some of the applications where multi-layer extrusion technology has been employed successfully on its own or in combination with other technologies.
Delivery Systems for Transcatheter Valve Replacement
Percutaneous replacement of diseased heart valves is an emerging therapy that offers an alternative to the traditional surgical approach for high-risk patients. In this minimally invasive procedure, a guide wire is placed through the femoral access site (in the groin), similar to an angioplasty, and guided into the chambers of the heart. An introducer sheath is inserted over the guide wire, and the valve delivery system is then introduced. Once the replacement valve is located in the correct position inside of the diseased native valve, it is deployed. The patient is fully conscious throughout the minimally invasive procedure.
The tubing required to deliver the valve to the site is a highly specialized component. The distal end must be easily steerable so it can be guided directly into the chambers of the heart. The tubing needs to be pushable, and thus, the proximal end needs to be more rigid. It must also have excellent kink resistance, and the inner diameter needs to be lubricious for smooth, unobstructed delivery of the replacement valve. Another key requirement is that the tubing wall must be as thin as possible to maximize the delivery channel diameter for the replacement valve without the tubing losing any of its functionality.
(Above) Braiding laid over transparent, thin-walled tri-layer and laminated with varying durometer sheaths.
In designing this particular component, the designers faced a number of challenges. Polytetrafluoroethylene (PTFE) lubricious liners, which are often the industry standard for delivery systems, could not be used due to concerns about the effects of gamma radiation during the sterilization process. Other concerns included attaining PTFE liners with the ultra-thin walls necessary for this application and also achieving a good bond with the other materials in the component. In order to ensure the tubing did not kink or deform as it navigates through the anatomy to the heart, it was necessary to incorporate a braided layer into the component. The distal end of the component needed to be more flexible for trackability, and the proximal end of the component needed to be less flexible for pushability.
The resulting design was a five-layer component, consisting of an ultra-thin-walled multi-layer tube, a layer of stainless steel braid, and various polyether block amide sheaths of different durometers on the outer layer of the component to increase the overall flexibility from proximal to distal end.
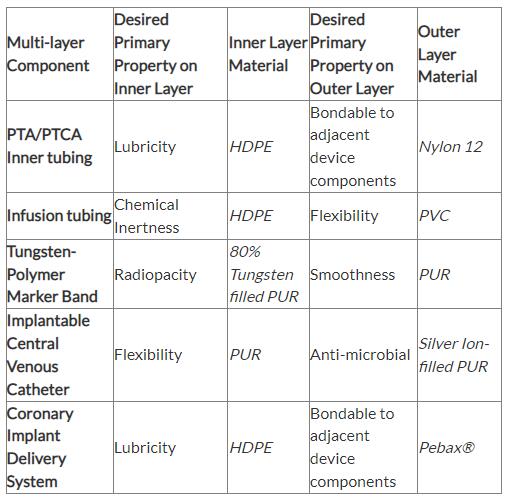
The multi-layer tube employed on the inner diameter of the component consisted of high-density polyethylene (HDPE), a tie layer and polyether block amide, with a wall thickness of less than 0.008 inches and an outer diameter ranging between 25 Fr and 35 Fr. HDPE was used on the inner layer for its low friction properties. The outer layer was polyether block amide to give an excellent bond with the outer sheath tubes, ensuring no delamination across the braided layer. The stainless steel braided layer provided the necessary structural rigidity and kink resistance. By using a multi-layer instead of several single-layer tubes, the wall thickness could be kept to a minimum, and assembly time and costs were reduced.
Percutaneous Transluminal Coronary Angioplasty
Percutaneous transluminal angioplasty (PTA) and percutaneous transluminal coronary angioplasty (PTCA) are performed to treat vessel narrowing. In both procedures a wire is passed from the femoral artery in the groin (or, at times, from the radial artery or brachial artery in the arm) to beyond the area of the artery being treated. A balloon catheter advances over the wire to the segment that is to be treated. When the balloon is inflated, it compresses the plaque and stretches the artery wall to expand, improving blood flow. During the procedure a stent may also be placed at the site of the occlusion.

(Above) Microbore multi-layer tubing for PTA and PTCA applications shown next to the eye of a sewing needle. Photo courtesy of Vention Medical.
Microbore multi-layer tubing can be used to make the inner shaft tubing of PTA and PTCA catheters. Using a multi-layer extrusion in this application, allows the finished catheter to have enhanced performance characteristics while reducing overall wall thickness to less than 0.002 inches in some cases. The inner shaft is typically a three layer tube consisting of a lubricious material on the inner layer, usually HDPE, to allow for ease of passage over a guide wire. Polyamide or polyether block amide is used for the outer layer because of its weldability with other components in the device. The middle layer consists of a tie or bonding material used to join the incompatible inner and outer layers of material. With recent material developments, it is now possible to use lubricious inner-layer materials that are compatible with the outer-layer materials. These materials can be in the form of lubricity-enhanced polyamides or fluorocarbon-based materials—there is no longer a need for a tie layer sandwiched between the outer and inner layers.
By using materials in the middle layer to change the mechanical functionality of the tubing rather than merely bonding the inner and outer layer, the possibilities become almost endless for the designer. Additionally, multi-layer technology is increasingly employed to manufacture other tube components within PTA and PTCA devices, such as balloon tubing and outer-body tubing. Using multi-layer tubing with an incorporated stiff middle layer, overall wall thickness can be reduced significantly or, alternatively, by maintaining the wall thickness, burst resistance could be increased over that of a single-layer tube.
Infusion Tubing
Infusion tubing is used to deliver drugs to the body. Traditionally, flexible PVC, which contains plasticizers and other additives, was the material of choice for this application because of its low cost, excellent anti-kink properties, ease of processing, and assembly using solvent bonding. However, PVC is not compatible with insulin, nitroglycerine and oncology drugs such as paclitaxel. The active ingredients of such drugs adsorb into the PVC tubing, resulting in a loss of potency, and only a portion of the desired dose reaches its target. More hazardously, the infusion solution can dissolve the plasticizer and other additives contained within the PVC, which inevitably end up in the patient.
Polyethylene, because of its inertness, has excellent compatibility properties, but is difficult to bond and has poor anti-kink properties. Multi-layer tubing containing low-density polyethylene (LDPE) on the inner diameter for compatibility, and PVC on the outer diameter is the ideal solution. The LDPE layer acts as a barrier, ensuring there is no loss of active ingredients through the absorption of the infusion solution, or contamination due to migration of additives within the polymer material. The outer layer of PVC ensures kink resistance is optimized and that the tubing can still be assembled by solvent bonding, packaged and sterilized in the same way as standard infusion tubing. Chemical compatibilities such as this are common challenges that can be easily overcome with the advancements in multi-layer tubing technology.
The advances in multi-layer tubing have come a long way in providing increased capability to procedures that were previously difficult to execute. The versatility of materials used in multi-layer tubing creates virtually endless combinations that can easily overcome medical challenges—and still remain competitive in the market. The innovative use of multi-layer tubing in medical design has revolutionized the way we solve a plethora of medical problems. In the future, as extrusion-tooling and machine-design technology becomes more advanced, combined with improvements to process control and measurement systems, we hope to see even greater precision, with smaller, thinner and more functional multi-layer tubing. Over time, multi-layer extrusion lines will become more specialized and incorporate a combination of other extrusion technologies, such as multi-lumen extrusion, tapered extrusion, “Over the Wire” extrusion and interrupted/intermittent layer extrusion.
Article source: MPO









