Pioneering the Future of Healthcare: Meet the Trailblazing 2023 Medical Finalist and Honorable Mentions
The 21st annual “Create the Future” Design Contest for engineers, students, and entrepreneurs worldwide, sponsored by COMSOL, Inc., and Mouser Electronics, drew more than 500 innovative product ideas from engineers and students in from 60 countries from around the world. The Medical category itself received 54 innovative entries from 22 countries. Analog Devices and Intel were supporting sponsors, and Zeus sponsored the Medical category. The contest, which was established in 2002, recognizes and rewards engineering innovations that benefit humanity, the environment, and the economy.
Finalists were selected from the seven categories: Aerospace & Defense, Automotive/Transportation, Consumer Product Design, Electronics/Sensors/IoT, Manufacturing/Robotics/Automation, Medical, and Sustainable Technologies/Future Energy.
In addition to product ideas at the concept or prototype stage, contestants could submit designs for commercial products introduced to the market within the last 12 months.
A competition among the finalists from all categories took place November 10, at which the grand prize winner was selected by a panel of judges. The grand prize winner received $25,000, while the first-place winner in each category received a Hewlett-Packard workstation computer. This article introduces the Medical Category finalist as well as those who received honorable mention. Congratulations to all who entered.
A NEW TREATMENT APPROACH FOR SPINAL CORD INJURY
Motivation. Spinal cord injury (SCI) afflicts 17,000 Americans and 700,000 people worldwide each year. Prognoses are often tragic, and social costs exceed $2 million (US and EU) in the first five years of care. For decades, the dream of repairing the spinal cord has been elusive. Recent studies, however, show that new axonal growth and formation of functional circuits are possible under the right conditions: a favorable biophysical environment in direct contact with surviving nerve endings. Chances for recovery increase greatly if such an environment can be provided rapidly after injury.
NeuroPair’s fast and minimally invasive approach generates an injectable scaffold of aligned magnetic nanoparticles to create conditions for protecting, healing, and regrowing neurons that were damaged during SCI. The scaffold guides the direction of neuronal regrowth and may help enable recovery from SCI.
Because shape and condition of SCIs vary so much, one-size-fits-all approaches are very unlikely to succeed. NeuroPair’s injectable hydrogel formulation addresses these needs by perfectly filling any irregular shape of the injury site, generating a flexible fiber scaffold with cellular contact for neuronal regeneration within minutes, suppressing scar formation, and being compatible with other treatment options (such as stem cells, neuroprotective and bioactive growth factors, epidural stimulation, and MRI). No other approach like this exists.
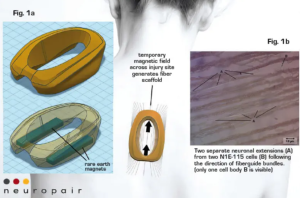
Methods. NeuroPair’s Fibermag™ treatment is applied after the initial, standard stabilization of SCI: The surgeon injects the sterile formulation into the injury site and places a magnetic mouse directly on top of the injury site. The magnetic field assembles the particles into a nontoxic and biodegradable scaffold (see Figures 1a and 1b). After five minutes, the field is removed, the scaffold is in place and remains stable yet flexible for weeks. The guided regrowth of axons and neurites along the magnetic fibers can be seen in vitro by brightfield and fluorescence microscopy over the course of several days and weeks. The Fibermag formulation consists of commercially available core components: biocompatible magnetic nanoparticles and slow-release bioactive factors in a hyaluronic acid-based gel.
Market. Many SCI patients are lifelong recipients of Medicare and Medicaid support, leading to U.S. social costs exceeding $51 billion each year. By restoring patients’ quality of life and functionality, these products can potentially reduce U.S. healthcare costs by 3 percent.
NeuroPair’s products could be easily integrated into treatment by neurosurgeons who presently treat SCI. The key elements of the technology that neurosurgeons find attractive are: 1) provides an injectable scaffold, 2) conforms to the irregular shape of an injury, 3) suppresses scar formation, 4) provides a favorable environment for regrowth, and — most importantly — 5) is fast and minimally invasive (no surgical resection of the cord is needed).
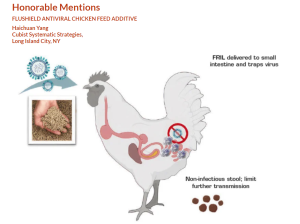
Avian influenza is a growing global problem. It seriously threatens the livelihood of poultry growers by killing domestic poultry such as chickens, ducks, and turkeys, etc. In the current avian influenza outbreak in the United States alone, over 58 million domestically raised chickens have been killed. Vaccines are banned in the United States and discouraged in global poultry trade by many countries due to unknown residue viruses in vaccinated birds.
In response to the situation, Cubist Systematic Strategies is developing a novel antiviral poultry feed additive, FluShield. The key component in the formulation is FRIL — an antiviral protein naturally present in L. purpureus (lablab bean). FluShield is both effective and safe: data has shown that using lablab bean extract, which contains FRIL, could neutralize high pathogenic avian strains H5N1 and H7N9 up to 100 percent and 96 percent, respectively; and since FRIL is a natural protein, the cost of sourcing raw materials and manufacturing FluShield is low.
FluShield’s patent-pending formulation preserves the natural form of FRIL proteins and slows down the degradation and removal of FRIL in avian digestive systems.
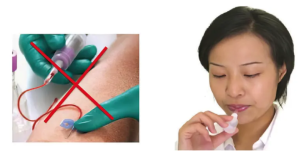
Neurodegenerative diseases require imaging and repeated testing, even though body fluid biomarkers are on the horizon. These are likely based on blood tests, which are impractical, somewhat invasive, and require medical personnel and equipment.
The goal of this project is to develop devices for rapid point-of-care measurement in the salivary fluid of the brain-derived protein analytes in individuals at risk of neurological diseases. The company’s IP covers all brain proteins measurable in saliva with a molecular weight of less than 60 kD. The invention is based on the discovery of brain-derived protein analytes that will show up in saliva based on an algorithm developed by the company.
The Flocel test can be self-administered, similar to a COVID antigen-based test. This is the first study measuring salivary proteins that are brain-derived. The company has a prototype for a saliva-based POC test for S100B. This is the first attempt to manufacture a saliva test that can be used at home without specific training or need for intervention by medical personnel (consumer product). The test is based on immunodetection of the selected antigen in less than a teaspoon of saliva. Completion of the test occurs within 15 minutes.
The testing strip uses a lateral flow chamber design, similar to home-based COVID tests.
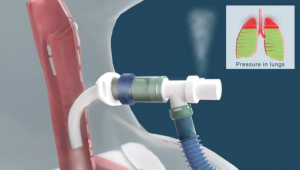
The Problem. Patients undergoing surgery may experience an accidental endotracheal tube disconnection from the anesthesia/ventilator breathing circuit. This dangerous accidental disconnect is known clinically as pop-off. When a pop-of disconnection occurs, the patient may suffer serious, permanent injury or death due to inadequate sedation and oxygenation.
Solution. The patented POPSafe™ Endotracheal Tube (POPSafe™ ETT) is a medical device that eliminates deadly accidental anesthesia/ventilator pop-off disconnection events. The POPSafe Endotracheal Tubes are the only products addressing the failure root cause of accidental anesthesia/ventilator breathing circuit disconnections.
The FDA 510(k) cleared endotracheal tubes include a patented ventilator elbow connector and patented over-pressure relief safety valve. POPSafe ETT is a next-generation endotracheal tube device intended to prevent dangerous, accidental anesthesia/ventilator pop-off events.
The company has developed initial POPSafe™ prototypes and conducted successful initial simulated clinical use testing. POPSafe is a next-generation endotracheal anti-disconnect device that will be GMP manufactured and FDA 510(k) cleared, similar to currently available endotracheal tube devices.
The iHeart Precision Oximeter measures aortic stiffness using the fingertip arterial pulse signal.
Aortic stiffness is known to be a surrogate measure of spinal flexibility and inner mobility. With youthful aortic stiffness, there is full mobility of the spine, rib cage, diaphragm muscle, and other core tissues allowing each breath to act as an engine of health.
With youthful inner mobility, each breath compressed and releases the abdominal internal organs promoting organ micro circulation and organ function, with each breath air fills the lungs and blood returns to the heart, with each breath cerebrospinal fluid is pumped up the spine to nourish the brain.
Aortic stiffness improves consistently with exercise, healthy diet, and stress management. Tracking aortic stiffness allows individuals to objectively recognize the benefit of making healthy lifestyle choices daily. The iHeart Precision Oximeter can measure aortic stiffness using the fingertip arterial pulse signal. Aortic stiffness measured with the iHeart Oximeter has been tested against gold standard aortic stiffness measurement systems and correlates well.
Article Source:Medical Design Briefs









