Visit
5 reasons to attend Medtec China 2022
1.Access to more than 2,200 global suppliers of medical device design and manufacturing from Medtech World
2.Free access to advanced products/technologies/service for the medical design and manufacturing industry
3.Resolve key technical challenges and problems in the processes of medical product development and manufacturing
4.The opportunity to explore regulatory updates in China, the US and the EU
5.Access to market trends and forefront business opportunities
Get your free pass to Medtec China 2022, you will get:
1.100RMB show directory
2.Free admission for exhibition and conference (Specific seminars are excluded)
3.Lucky draw and free chance to join paid-conference
4.Network with the market leaders and building new business contact leads
Key exhibit categories include:
1. R&D and Design Services
2. Computing and Software
3. Materials
4. Adhesives and Adhesive Products
5. Components
6. Eletronic Components
7. Motors and Motion Control
8. Pumps and Valves
9. Filters and IV Products
10. IVD
11. Cleanrooms and Environmental Control
12. Manufacturing Equipment
15. Tubing and Extrusion
16. Surface Treatment
17. Pharmaceutical equipment and services
18. Testing, Metrology, Inspection and Calibration Equipment & Supplies
19. Printing, Labeling, and Bar Coding
20. Packaging and Sterilization
21. Consultants
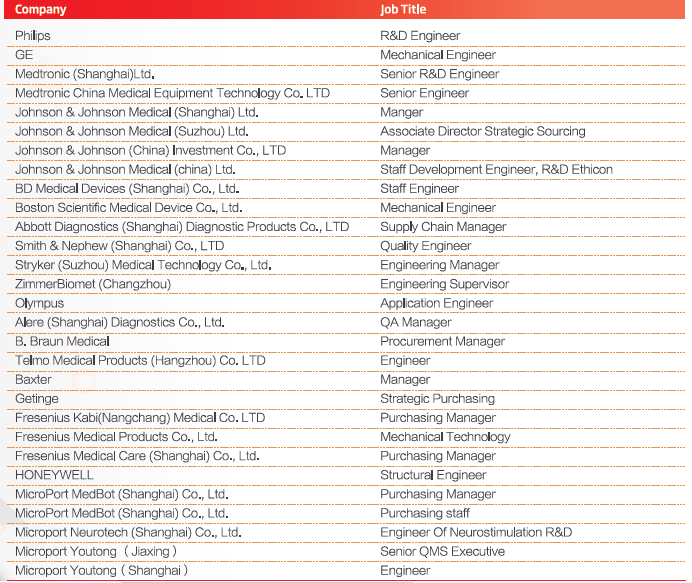
2022 Partial Exhibitor List


Who visited Medtec China?
MDiT Forum and Regulation Summit 2022:
Regulation Summit
• Chinese Regulatory Updates and Compliance
Quality Summit
• Quality Track A: Advanced Medical Device Life Cycle Risk Management
• Quality Track B: How to meet the quality requirements of products in overseas markets under the epidemic
Technology Summit
• Technology Track A: Boosters for the Birth of Medical Devices-Innovative Medical Materials/Accessories and Precision Machining(Ⅰ)
• Technology Track B:Plastic Molding Technology in Medical Device Manufacturing
• Technology Track C:Precision machining equipment and Technology Forum
• Technology Track D:Overseas advanced medical manufacturing technology
• Technology Track E:3D materials and technology application in Medical Device
• Technology Track F:The 6th Conference of Medical Device Design
• Technology Track G:The 8th IIMD China Summit-Implantable and Interventional Medical Device
• Technology Track H:Technology Development of New Type Medical Dressings
• Technology Track I: Seminar of advanced Technology of Medical Bonding and Welding
• Technology Track J:Advanced Active Medical Device Technical Fourm
• Technology Track K:The 5th Session of Pack&Ster Hub
• Technology Track L:Boosters for the Birth of Medical Devices-Innovative Medical Materials/Accessories and Precision Machining(Ⅱ)
• Technology Track M:Quality Focus – Medical device life cycle risk management
• Technology Track N: The 4th Advanced Automation Medical Device Manufacturing Technology Forum
• Technology Track O: Core component and technology Seminar of dental products
Concurrent activities- An efficient interacting platform for exhibitors and visitors
Market Report Track of Medical Device Industry
In order to serve the needs of visitors to capture industry trends, Medtec China always invites consulting companies such as L.E.K and Medisophy Capital to the show to share the latest market reports covering the medical device industry, the entire medical industry, and the new growth points and changes in the medical device market.
Regulatory Lecture
Regulatory Lecture focuses on “Best Practice for Product Compliance and Market Launch”. Keynote Speakers were from Ming Xin Cardiovascular Disease Hospital of Wuxi, WuXi AppTec China Testing Center, and Mid-Link Group Company sharing their insightful ideas.
Quality Focus
Medtec China has a co-located show, Quality Expo, for establishing a resource exchange platform for people working in quality control. It usually gathers many high-quality testing companies such as Techmax Info. Tech. Co., Ltd.,Uson L.P., Boyue Instruments (Shanghai) Co.,Ltd,Tinius Olsen Testing Machine Shanghai Co., Ltd,Ningbo Enorsen Intelligent Manufacturing Co.,Ltd,MARPOSS (SHANGHAI) TRADING Co., Ltd,and Innomatec China to exhibit, as well as hold a Quality Expo conference focusing on quality control, which provided a professional communication platform for quality engineers.
Exhibitor Seminar
Celanese (China) Holding Co., Ltd
Addressing the Needs in Drug Delivery Devices -Material and Technology innovations from Celanese DuPont China Holding Co., Ltd.DuPont TM Liveo TM New Brand Launch









