Electronics Integration into Disposable and Single-Use Medical Devices
The medical device market has been rapidly changing over the last decade and a key area of change is addressing today’s fast paced, data-driven environment. Multiple sources state the digital health market to currently be an over $5 billion industry with growth predictions to be over $90 billion by 2025. Factors in the growth of electronics integration into medical devices range from the need for accuracy of the device performance in the surgical suite to an individual patient’s desire for monitoring their own health.

Medical device companies face many challenges in matching today’s technology desires while balancing strong industry cost pressures. Every feature and every benefit needs to be balanced against cost to result in a successful value-based solution. Integrating electronics into medical devices becomes even more challenging when the device is disposable. Single-use and single-patient devices, however, can have different considerations. A single-use disposable product is a one-time use, such as a hand-held surgical device requiring electronic integration to provide and monitor balloon pressure. A single-patient disposable is used multiple times by (or for) the same patient—examples include an inhaler or injectable pen. Both can be successful utilizing integrated electronics. In balancing features and cost for either application, a good place to start is the design.
Design Considerations
Designing or even redesigning an existing product to include electronics requires an understanding of user needs, available technology, and technology limitations, as well as the complicated maze of regulations that control these products.
Regulations for electronics in medical devices vary greatly from their non-electronic counterparts as well as from country to country. When the new product development calls for an electronic/electro-mechanical solution, the following elements should be considered.
Human Factors
Integrating electronics may open vast possibilities for the human interface but careful consideration must go into understanding the target user. This is no longer just a common-sense thing to do; it is now required by the FDA. ISO 13485:2016 explicitly references Usability Standard IEC 62360, which is a part of the overall compliance to IEC 60601. IEC 62360 is harmonized by the European Union as well as the United States and can be used to satisfy the regulatory requirements of both markets.
Battery Life
Battery life can be the most challenging obstacle in implementing a disposable or single-use medical device with integrated electronics. This is compounded further if the device also needs RF communication or a display with backlight. Advances in low-power microcontroller architecture and higher density battery technologies have opened up new opportunities.
Environmental Concerns
Ambient light or backlight, temperature, humidity, storage conditions, and chemical exposure all play a role in the component selection and the sealing requirements (IPXX).
Interface Requirements
While many devices only provide user feedback via indicators or displays, some may require communication to another device like a smart phone, access point, or directly to the cellular network for communication to a distant location for remote monitoring. Direct cabling has largely been displaced by wireless communications via RF or IR links. IR links offer lower cost and less stringent regulatory requirements but are limited to line of sight communications and are directional. RF communications offer much wider options and that brings in the challenge as discussed next.
RF Communications
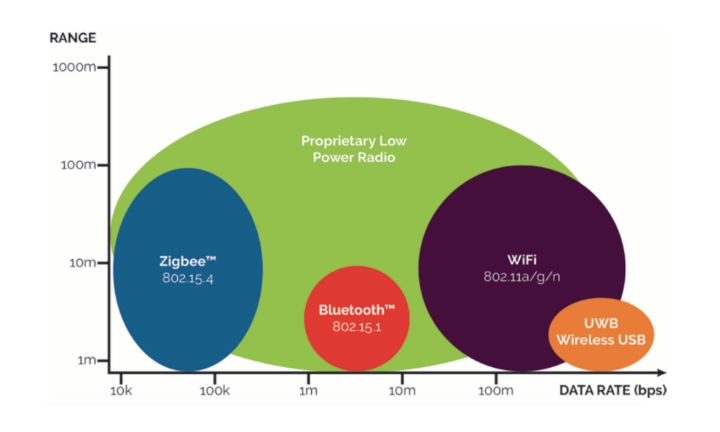
International standards vary widely in both the frequencies allowed as well as power level, so the device destination is needed early in the design phase. A comprehensive study of the international frequency allocation is necessary. In the U.S., there is a specific frequency allocation for implantable (400 Mhz) and Wireless Medical Telemetry (WMTS: 608-614 Mhz, 1395-1400 Mhz, 1427-1432 Mhz—mostly U.S. only). Devices that are placed within 20 cm of the patient require routine monitoring to demonstrate compliance with the FCC radiation exposure guidelines. Once the market homework has been completed the RF protocol must be decided. This can vary from a completely dedicated design and proprietary protocol to one of the popular short distance communications protocols such as Zigbee or Bluetooth and midrange communications such as Wi-Fi which operate in the ISM band (Industrial, Scientific, Medical: 902-928 Mhz and 2.4-2.5 Ghz). For longer distance communication, direct cellular connection has become the most popular. Battery life considerations will likely rule out cellular for a disposable device. Short-range communications in the 2.4 GHz frequency range offers many benefits such as low power, noise immunity, and widely available modular components that make integration much less challenging and worldwide homologation. Zigbee offers a high reliability, low cost, and low power communication option but at lower bandwidth than Bluetooth. Bluetooth is a widely used standard in portable devices such as cell phones. BLE (Bluetooth Low Energy) was introduced in 2006 under the name Wibree and merged into the Bluetooth standard V4.0 in 2010. BLE targets very low power applications utilizing coin cells. Today BLE is marketed as Bluetooth Smart (Figure 1).
Embedded Antenna
The antenna used for your RF design may or may not play an important role in the overall device performance depending on the distance requirements. The farther the communications needs, the more critical the antenna design will be. Space considerations often are a major handicap for a proper antenna implementation lacking suitable ground plane and physical length to achieve a full ¼ wavelength. At 400 MHz, the antenna should be approximately 7 inches long. While at 2.4 GHz the antenna length can be approximately 1.2 inches. Many board-mounted antenna are commercially available, but again space considerations often make them impractical leaving the engineer with a PCB trace as the antenna solution. If performance is critical, engage an antenna design consultant with experience in embedded antenna design.
Regulatory
All devices with a clock circuit must pass FCC/EMI/RFI testing and be included as part of your 510(k) submission. Most of this is covered in the IEC 60601 standard, but be aware that part 11 of the standard deals with home use devices and places even more stringent requirements on emissions. Devices that contain RF communications must be further qualified by the FCC and carry an RF device ID. Your compliance requirements can be greatly lessened by integrating RF modules with a recognized module ID. With this, only RF screening with the production equivalent antenna is needed for filing with the FCC.
Board Classification
Quality standards exist for PCBAs and the classification specified. This will have an impact on the cost of the board and therefore the product. High reliability products that may be used in life support are classified as Class III. Most medical devices fall into a Class II requirement allowing the PCBA manufacturer more latitude in their inspection criteria, which can reduce manufacturing costs.
Software/Firmware
The vast majority of intelligent medical device recalls are due to software issues. As a result, there are very stringent requirements on the development and testing of all elements in the software development process from module testing (referred to as Unit Testing), integration testing, and finally complete system testing. This overhead to the development process is significant, but a necessary part of creating software design resulting in a robust, intuitive and reliable finished product. FDA, IEC, and ISO standards and guidelines apply.
Manufacturing Considerations
Medical devices containing electronics also face strict adherence to device integrity and compliance when being manufactured. Process repeatability and validation, material component analysis, regulatory, shelf life, device handling due to ESD issues, supply chain management, and assembly all are factors to be addressed prior to the manufacturing stage. Manufacturing considerations when dealing with electronic integration include:
Medical devices with electronics often have a shorter shelf life than their non-electronic counterparts. The FDA’s regulations and policies relating to the shelf life of medical devices identify parameters that determine the stability of the device over time. Identifying shelf life early allows for accuracy in the timeline for manufacturing, storage, shipping, and distribution to the end user, and avoids further demands and tests.
Process repeatability and validation are critical to manufacturing medical devices. The development of an automated production line can be a key benefit: a repeatable validated process, precise assembly methods, less “human” touch, and overall product quality improvement.
When handling and assembling devices with electronics, manufacturers need to understand ESD issues. Sensitive medical devices must be supplemented with ESD control measures for in-coming inspection, in process handling and assembly, and final packaging.
Sterilization processes can have deleterious effects on electronics, so understanding the effects on the device as well as the electronics is vital in choosing the sterilization process. As a general guideline, Gamma and E-beam sterilization damage the electronics. Autoclave and ETO processes can limit the function and battery life. It is imperative to work closely with sterilization services so they fully understand the user requirements of the device so the appropriate sterilization method can be chosen.
A traceability plan, depending on the final device use, needs to be put in place. Product needs vary from lot traceability to RFID tagging. Traceability is required with all combination devices containing any type of pharmaceutical.
A careful analysis of the electronics supply chain is critical for sustainable manufacturing and meeting time to market requirements. Many manufactures of largely mechanical devices fail to appreciate the lead-time challenges of some electronic components, which can be as long as 39 weeks. Single sourcing of critical components is an often-overlooked liability issue. This is easy to deal with for commodity components but not nearly so easy with specialized components, which may only have one source. Upfront planning is required to identify long lead items to fill the product pipeline prior to your intended launch. Also, any second source that is chosen will require additional validation.
It is not uncommon for an electronic component to become obsolete by the manufacturer. Usually there is a recommended alternative component but the new component will have to be validated within the design, which takes time and resources. Proactivity on the part of the contract manufacturer and the OEM is required to stay abreast of the anticipated production life of all components involved.
Market Supply
Unavailable—what happened? On rare occasions, a component can be used on another high-volume device. Suddenly the entire market supply is unavailable overnight. While not common, when it happens, it creates a mad scramble to design out that component and revalidate. In addition to the cost implications for the boards, this scenario has additional costs associates with WIP and current stock. Another less common event is an act of nature that disrupts your supply chain. While these examples are rare, balancing the risks of supply along with the costs of early validation of more than one source should be considered.
Costs
A key consideration of electronic integration into disposable and single-patient use devices is cost. The marketing and business case will not be dealt with here, but it is fair to say that in most cases the electronic device will be more expensive than a purely mechanical alternative. Adding electronics specifically into disposable devices, where there is very strong cost pressure, may be justified by benefits such as enhanced device functionality, increased patient adherence, a needed dataset record, and information allowing better patient management of the therapy. Added to this is the consumer experience and expectation of personalized data, along with patient behavior changes when their personalized data is available to them. When devices go beyond a mechanical function to objective data identifying medication trends, the patient experience improves, the patient therapy should improve and this can result in cost savings in the healthcare system.
Cost assessments of most single use disposable devices often use conventional metrics when considering mechanical or electronic features, such as annual volumes, bill of material costs, etc. Additional factors, such as a specific patient benefit or reducing the time of a surgery will influence the outcome of a purely device-to-device comparison. Reviewing the cost of the entire medical system can change the direct-to-direct comparison.
Combination devices and devices that are single-patient use can allow greater success in justifying electronic features as the device itself is used over a period of time before disposal.
Existing devices are under the same scrutiny for cost as new devices. Restructuring the device architecture can create a significant cost improvement over the easier method of planning for cost improvement by starting with the existing device. Over time, a customer’s desired features and perception of value shifts, and starting with those new perceptions of value can result in a device that more closely matches the market need.
Conclusion
Single-use disposable devices face greater challenges in satisfying the growing market for electronics integration given industry cost pressures. Whether the device is a one-time use disposable or a single patient multiple use disposable, a case can be made for the use of electronics even in traditional mechanical devices. Understanding the value of design to create an innovative solution that works together with strong manufacturing processes and strong supply chain relationships, can result in a device that delivers exactly what the customer wants at the best possible cost. Medical device companies that find balance between needs of the customers and cost pressures in devices that include electronics will find success.









