How Abbott created its next-gen RF ablation system
For Abbott, combining two innovations into one AFib-treating system required manufacturing fine-tuning. The project’s R&D director explains.
Pulsed field ablation (PFA) has generated a great deal of buzz for its potential to reduce complications in procedures for treating atrial fibrillation (AFib). But even as Abbott is developing its Volt PFA system — announcing in January that it kicked off a CE mark clinical trial of Volt — the medtech giant is betting that tried-and-true radiofrequency (RF) ablation can still make a big difference with the right innovations.
Abbott last year won FDA approval for its next-gen TactiFlex Ablation Catheter, Sensor Enabled. The company described it as the world’s first ablation catheter with a flexible tip and contact force technology.
“Launching TactiFlex — which is the flexible tip combined with the contact force — we’ve seen great results, great outcomes, whether it’s outcomes for the patient or time of procedure. We’ve seen that consistently around the world,” Abbott CEO Robert Ford said during the company’s fourth-quarter earnings call in January.
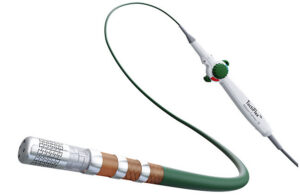
Abbott’s TactiFlex ablation catheter [Image courtesy of Abbott]
Erich Stoermer became director of R&D for electrophysiology (EP) catheters at Abbott in mid-2019, about a year after formal TactiFlex product development started. During an interview late last year with Medical Design & Outsourcing, he explained how the company already had contact force sensing in its TactiCath Sensor Enabled ablation catheter and a flexible tip in its FlexAbility Sensor Enabled catheter.
Each had benefits. Contact force sensing enables electrophysiologists to ensure the tip has the appropriate force (measured in grams) against the cardiac tissue being ablated. Meanwhile, the flexible tip provided better maneuverability, more grip against the tissue because of its lattice-like structure, and more efficient saline irrigation at the ablation site.
“Physician feedback was, ‘We think it’d be really powerful from a safety and effectiveness standpoint to really combine these technologies into one catheter,’” Stoermer said.
Integrated with 3D heart modeling and electrical mapping from Abbott’s EnSite X EP system, the next-gen system could reduce procedure times and boost safety compared with previous generations of RF ablation tech.
A top challenge to get there involved fine-tuning and tighter tolerances in the manufacturing process.
“Anything that goes into this is in the millimeter or sub-millimeter range/tolerance. … There are parts of our manufacturing process where we’re controlling things to the nanometer,” Stoermer said.
The reason why the force-sensing/flexible-tip combination required tighter tolerances became apparent as Stoermer described how the tri-axial optical force sensor system in TactiFlex works.

Erich Stoermer, Abbott’s director of R&D for EP catheters [Image from Stoermer’s LinkedIn page]
He gestured to a diagram of the system outside the Minneapolis-area cleanroom where workers in cleanroom garb were assembling the TactiFlex system under microscopes:
“You can see the tip electrode being fixtured, and it’s been set very carefully into the distal end of the catheter. … Those three shaft electrodes we talked about at the distal end of the catheter [for ECG readings], those are being brought up, and then we bond the shaft to the tip electrode. You can see this is what we call the deformable body, so as forces are applied to the tip, the force is transmitted. This … body deforms, and it deforms by nanometers. You’ve got three fiber optics being set into a deformable body. Those three optical fibers are reflecting light, back and forth. And then as forces are applied, the reflection of that light is transmitted differently. That’s what tells the computer how much force is being applied in one direction. And that goes into our TactiSys box, which then tells EnSite how much force is being applied to the catheter. So this is the heart of the force sensing technology right here.”
The handle — which has some new design features to let a surgeon maneuver the catheter without having to look down from the EnSite screen — gets assembled on the proximal end. Electrical wires, shaft deflection wires, thermocouple wires, fiber optics and the irrigation lumen all route back from the tip through the catheter’s 8 Fr distal section and 7.5 Fr shaft with steel braiding and a polymer jacket.
Stoermer declined to go into much detail about what kind of tweaking was needed to ensure the sensing system worked with a flexible tip, citing proprietary information. But he said getting the design and manufacturing process right involved computer modeling of how the contact force sensing system and flexible tip would work together in RF ablation procedures, and then experiment-based adjustments.
“It took some fine-tuning of our design and manufacturing process controls to ensure that we got the same level of consistency and accuracy as we had on the TactiCath,” he said.
Tighter tolerances were crucial for the undisclosed contract manufacturer that laser cuts the TactiFlex’s platinum iridium flexible tip.
“It’s largely automated, and that really takes care of providing a consistent high-quality component. … The stock of material is placed into the spindle, it’s grabbed onto by a robot, it’s held there, and then it’s computer controlled,” Stoermer said.
The result is a next-gen RF ablation system with combined stability and safety to improve physician experience and patient outcomes, according to Dr. Christopher Piorkowski, chief medical officer and divisional VP of medical affairs for Abbott’s electrophysiology business.

Abbott Electrophysiology Chief Medical Officer Dr. Christopher Piorkowski [Photo courtesy of Abbott]
“TactiFlex provides double the amount of stability at the moving heart wall, which can give physicians more peace of mind to make a good lesion in the moving heart of a breathing patient,” Piorkowski said. “The stability of the catheter plus the confidence in the position of the ablation work together to shorten the procedure time, reducing the risk of complications.”
Article Source: Medical Design & Outsourcing









