BD Gears up for Injection Device Demand for COVID-19 Vaccine
Pfizer created quite a buzz this week with news that its COVID-19 vaccine candidate was found to be more than 90% effective. Meanwhile, Becton, Dickinson and Company (BD), the largest U.S. supplier of injection devices, is ramping up to supply hundreds of millions of needles and syringes to support vaccination campaigns globally.
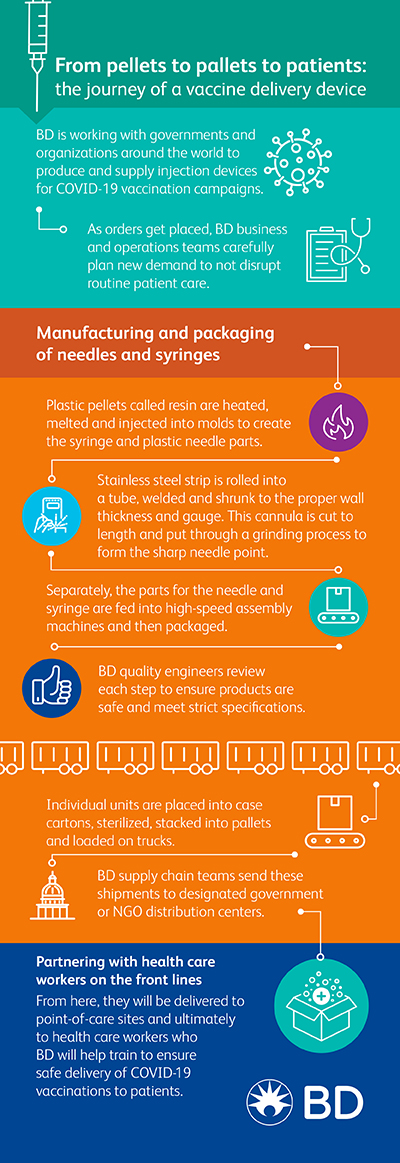
BD has committed more than 800 million needles and syringes to date in support of COVID-19 vaccination campaigns across 12 countries and non-government organizations – with discussions underway to provide millions more. But this is no simple task.
BD recently provided a look into the process of supplying injection devices for a COVID-9 vaccine when the time comes, using the U.S. market as an example. The U.S. government has placed orders for 286 million needles and syringes from BD, with 256 million to be supplied by January, the company said.
Planning demand to protect patient care
BD said it produces billions of needles and syringes for the U.S. market every year, but only a fraction of those devices are used to deliver vaccines. The company’s manufacturing operations are setup to produce enough devices to meet existing demand to support routine healthcare delivery. In the case of new orders from the U.S. government, BD said its business and operations teams must carefully plan so they don’t disrupt ongoing patient care needs for programs like the annual flu vaccination and childhood immunizations.
Manufacturing and packaging of needles and syringes
After the planning phase comes the manufacturing and packaging phases.
“While it may seem simple, these are highly calibrated, highly sophisticated medication delivery devices,” BD said in its recent blog post.
Plastic pellets called resin are heated, melted and injected into molds to create the plastic parts that make up a syringe like the barrel and plunger rod. The plastic parts of a needle – the hub and shield – are made the same way. The needle is created using stainless steel strip that is rolled into a tube and welded. A metalworking process called drawing is then used to shrink the tube until it gets to the proper wall thickness and gauge (the outer diameter measurement), and then cut to length. These blunt metal tubes called cannula then go through a grinding process to form the sharpened point, used to penetrate the skin when delivering the vaccine.
Separately, the components for the needle (cannula, hub, and shield) and for the syringe (barrel, plunger rod, and stopper) are fed into high-speed assembly machines and the final products enter packaging machines. The individual units are placed into case cartons that are sterilized on site, stacked onto pallets and loaded on trucks. Along the way, BD quality engineers review each step of the process to ensure products are safe and meet stringent quality inspections and specifications.
Shipping finished products to U.S. government distributors
BD supply chain teams will then ship the devices to distribution centers for the U.S. government’s chosen COVID-19 vaccine distributor partner, McKesson. Next, the devices will be delivered to point-of-care sites across the country based on the government’s COVID-19 vaccine distribution strategy.
Interstingly, however, Pfizer does not plan to use McKesson to distribute its COVID-19 vaccine if approved by FDA. Instead, the company said it will use its own distribution system to deliver the COVID-19 vaccine directly to healthcare providers, according to a FiercePharma report.
BD has also promised to partner with frontline healthcare workers BD to ensure safe administration of COVID-19 vaccinations.