Policies & Regulations
Taking a Deeper Look at Device Certification and Recertification
Experts discuss how to prioritize medical and IVD devices under the new MDR/IVDR in Europe.

2019年3月26日
Key Medical Packaging Standard, ISO 11607-1/2 Published; More Regulatory Support on the Way
With the EU Medical Device Regulations just over one year away, this latest revision aims to help medical device manufacturers meet the regulation’s General Safety and Performance Requirements.

2019年3月22日
SO 80369: Frequently Asked Questions, Answered
Here are answers to some common questions about developing and testing small-bore connectors.
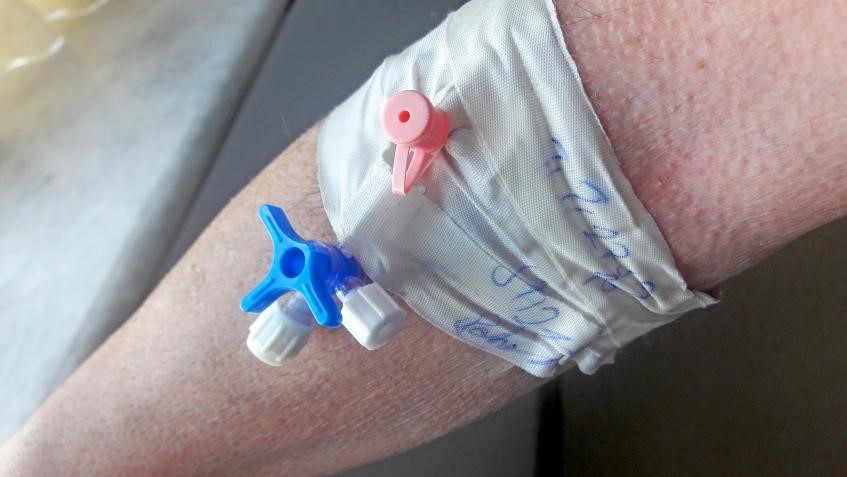
2019年3月14日
Another Reason to Update Your Biocompatibility Approach: EU MDRs
A chemist and biocompatibility expert offers the latest insights and predictions on medtech biocompatibility testing.

2019年2月19日
How Is the Government Shutdown Impacting Medtech?
FDA cannot accept new 2019 user fees during the government shutdown, which means the agency cannot accept new medical product applications.

2019年1月23日

